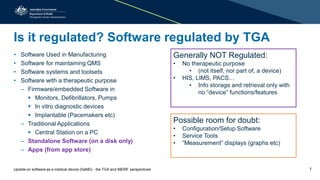
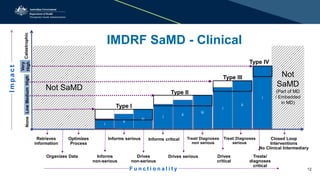
The document provides an overview of the regulation and definition of software as a medical device (SaMD) from the perspectives of the TGA and IMDRF. It outlines the criteria for determining if software qualifies as a medical device, the regulations surrounding its pre and post-market performance, and highlights challenges in classification and safety reporting. Additionally, it indicates that not all medical software is regulated by the TGA, focusing on those with therapeutic purposes and noting the importance of compliance with medical devices regulatory frameworks.