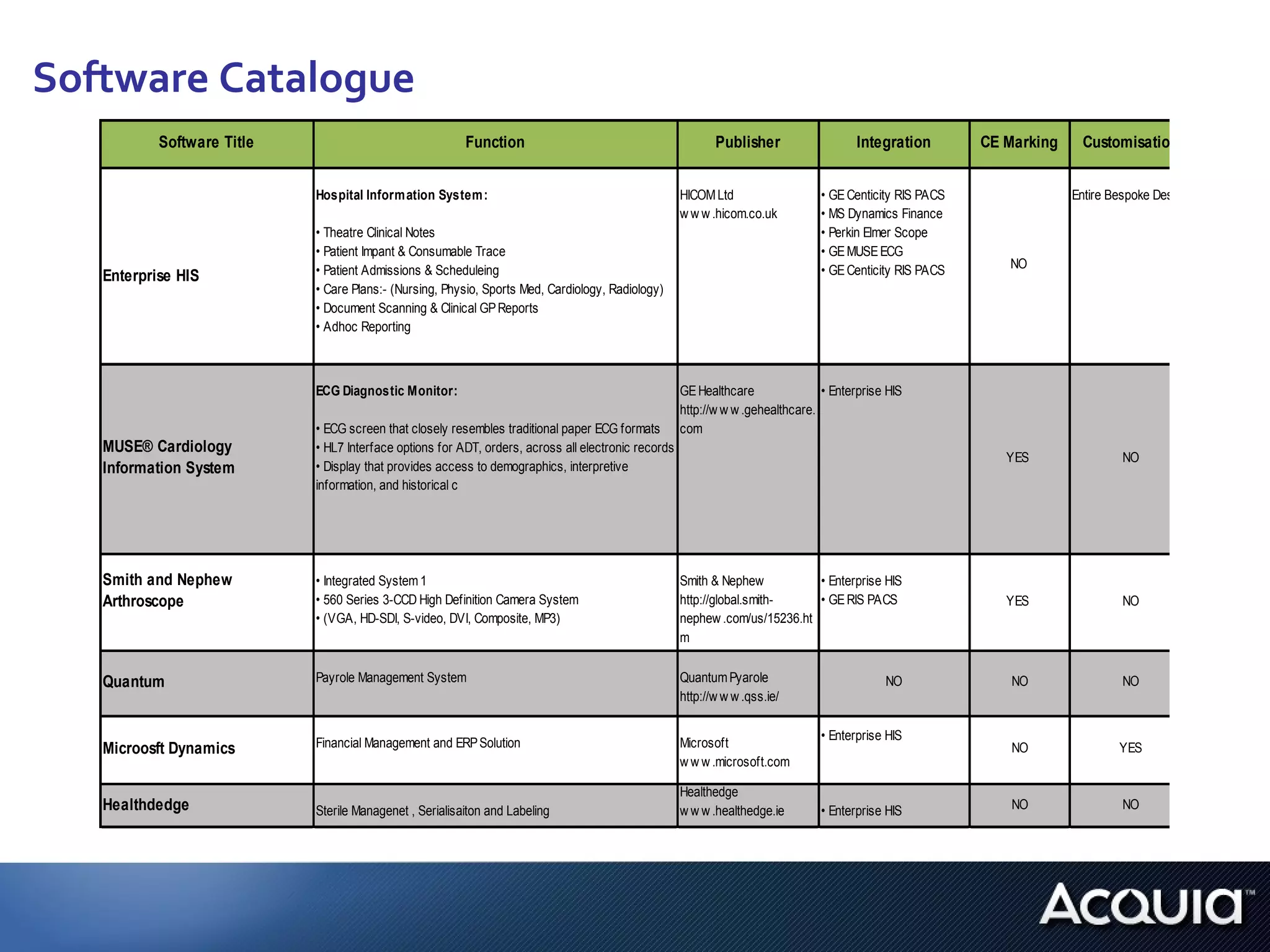
1. The document analyzes how software would be classified under the EU Medical Device Directive and the US Medical Device Data Systems regulation by applying them to a private hospital's software catalog.
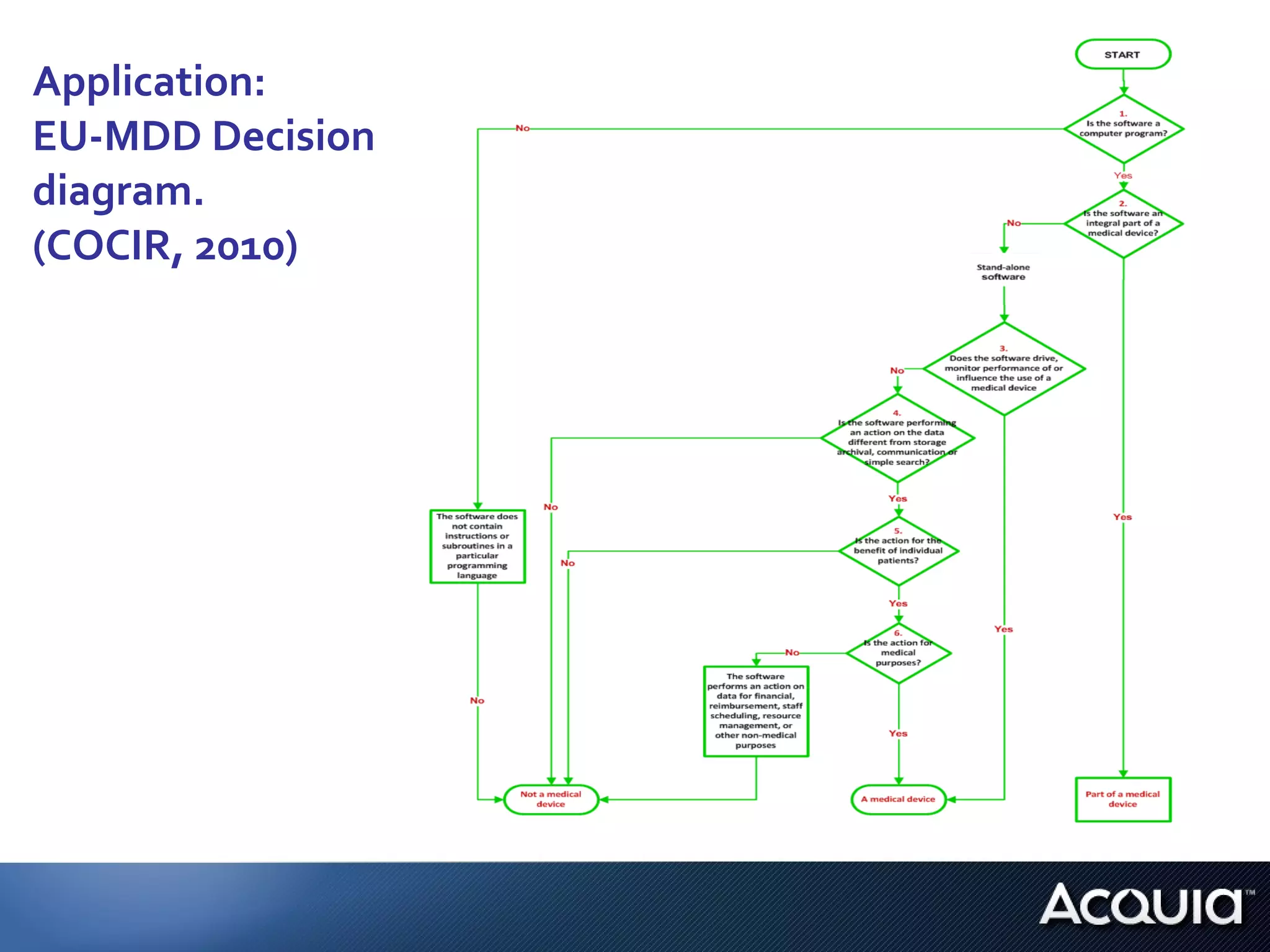
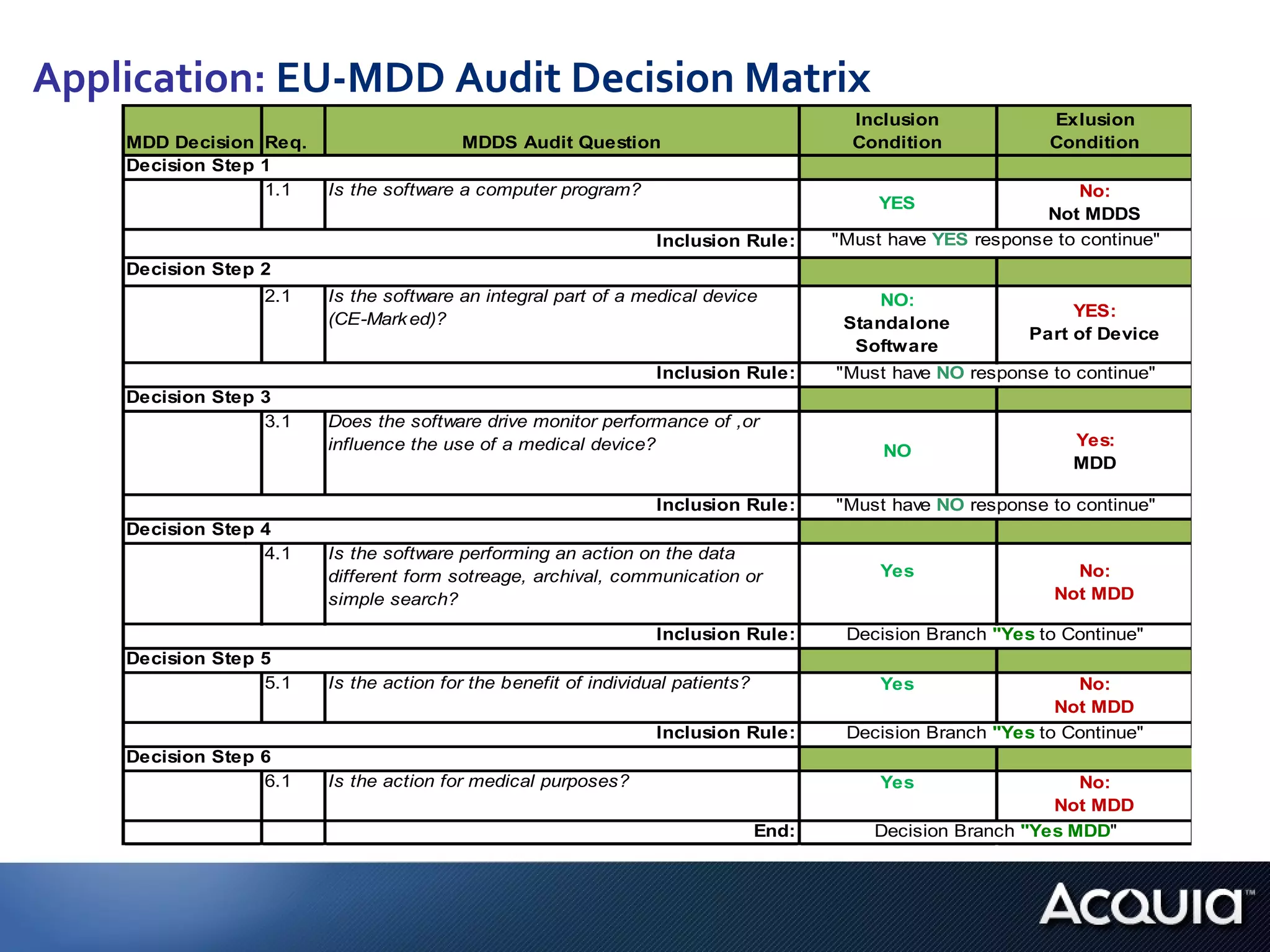
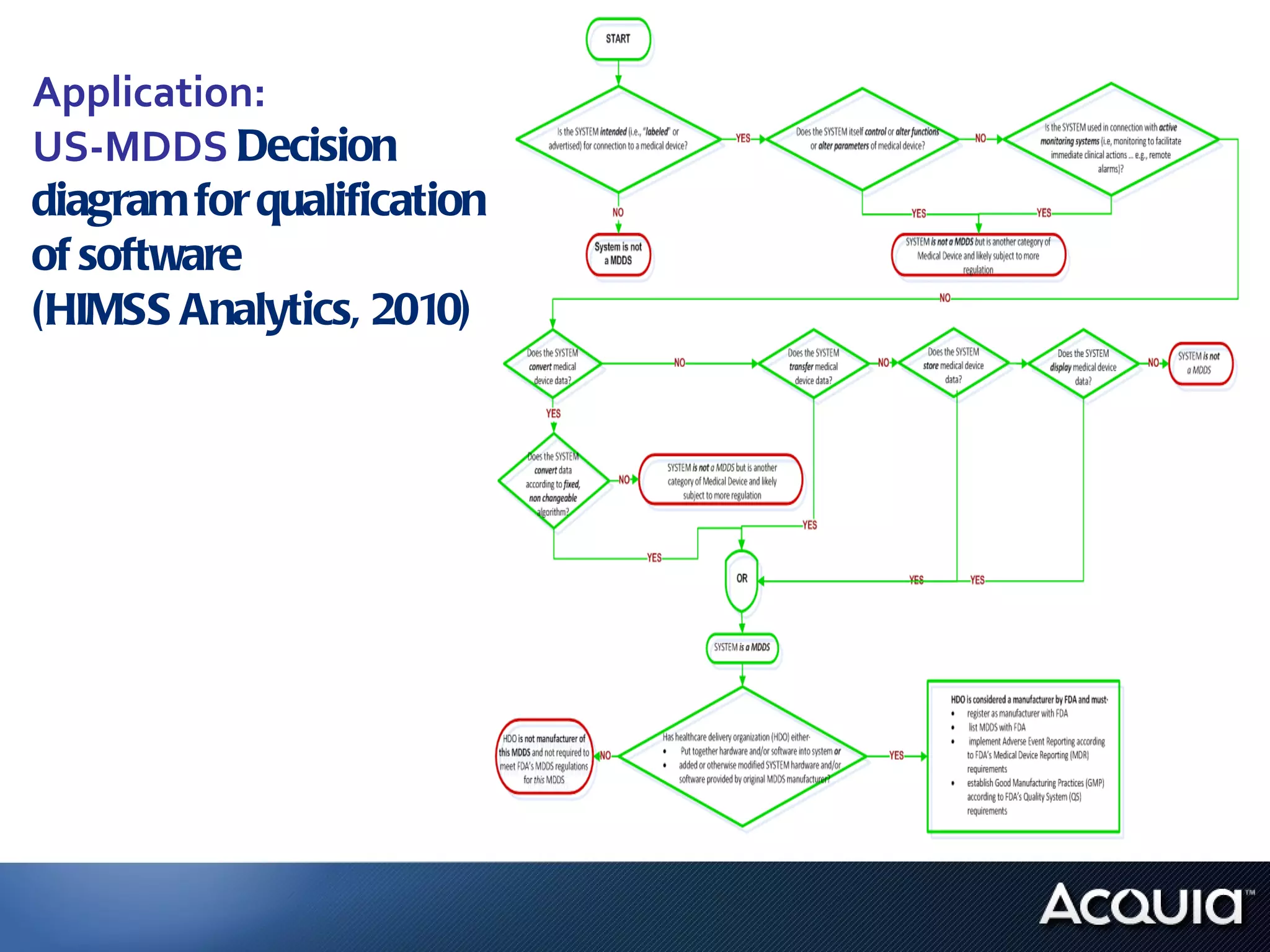
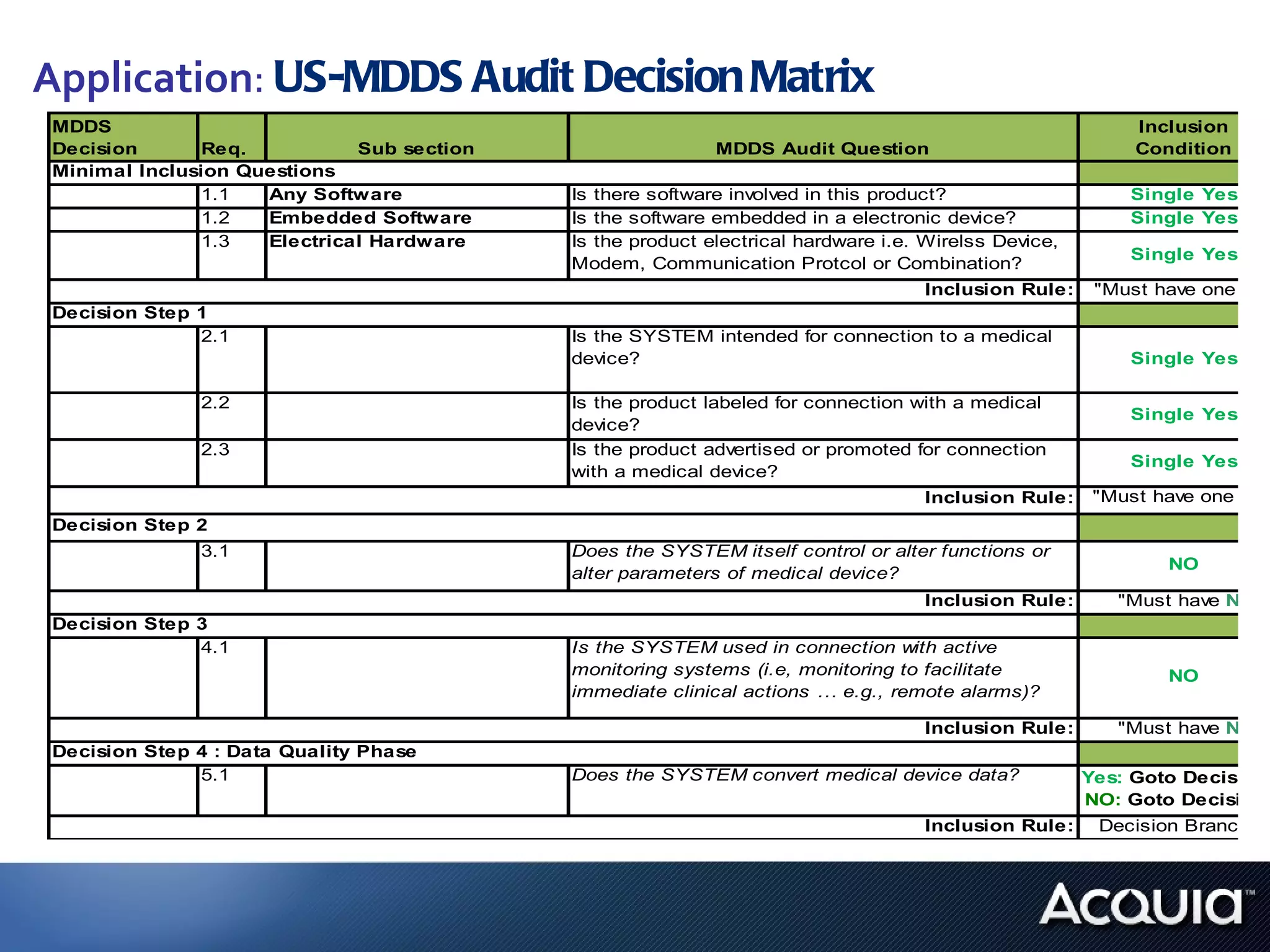
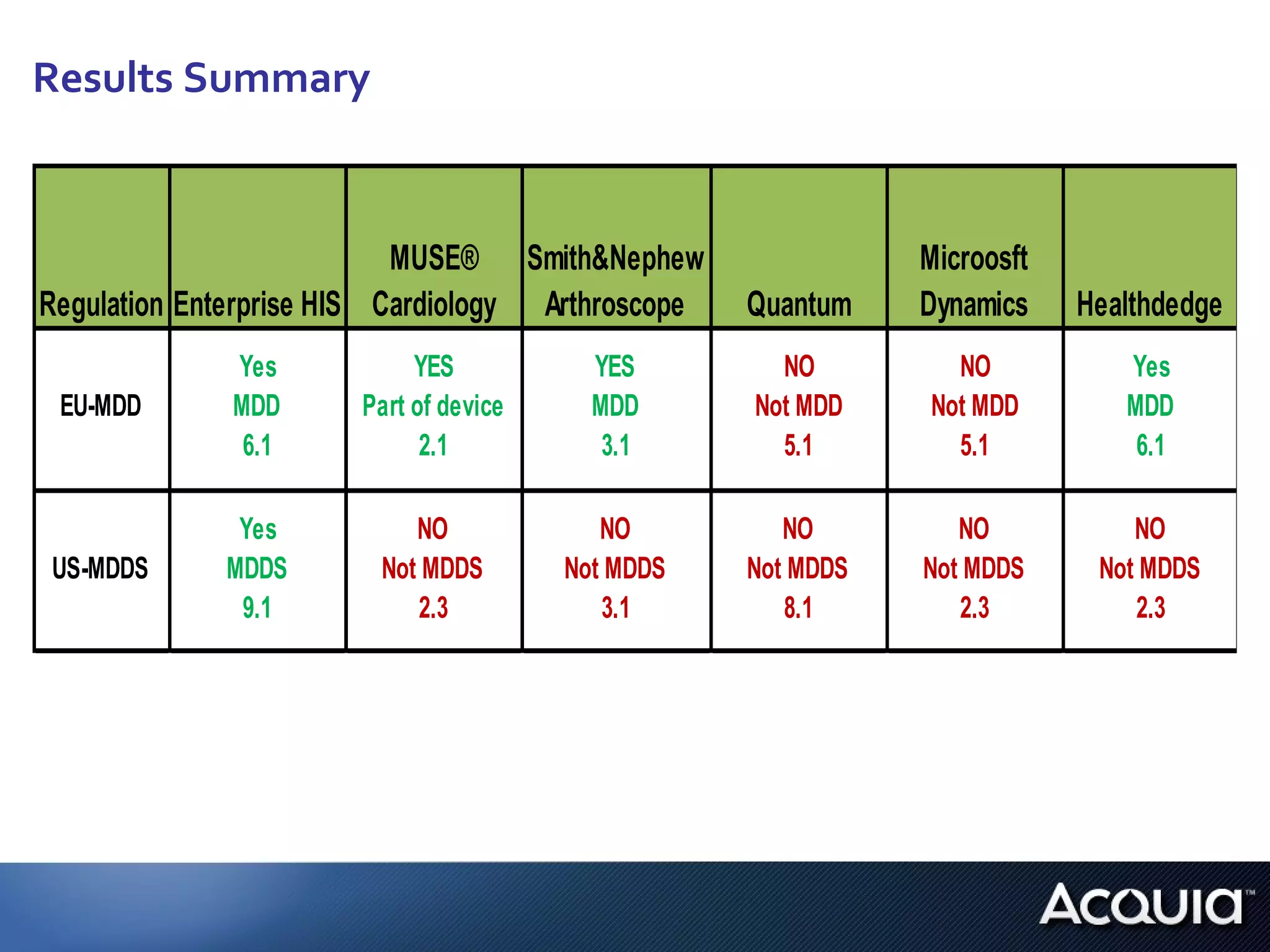
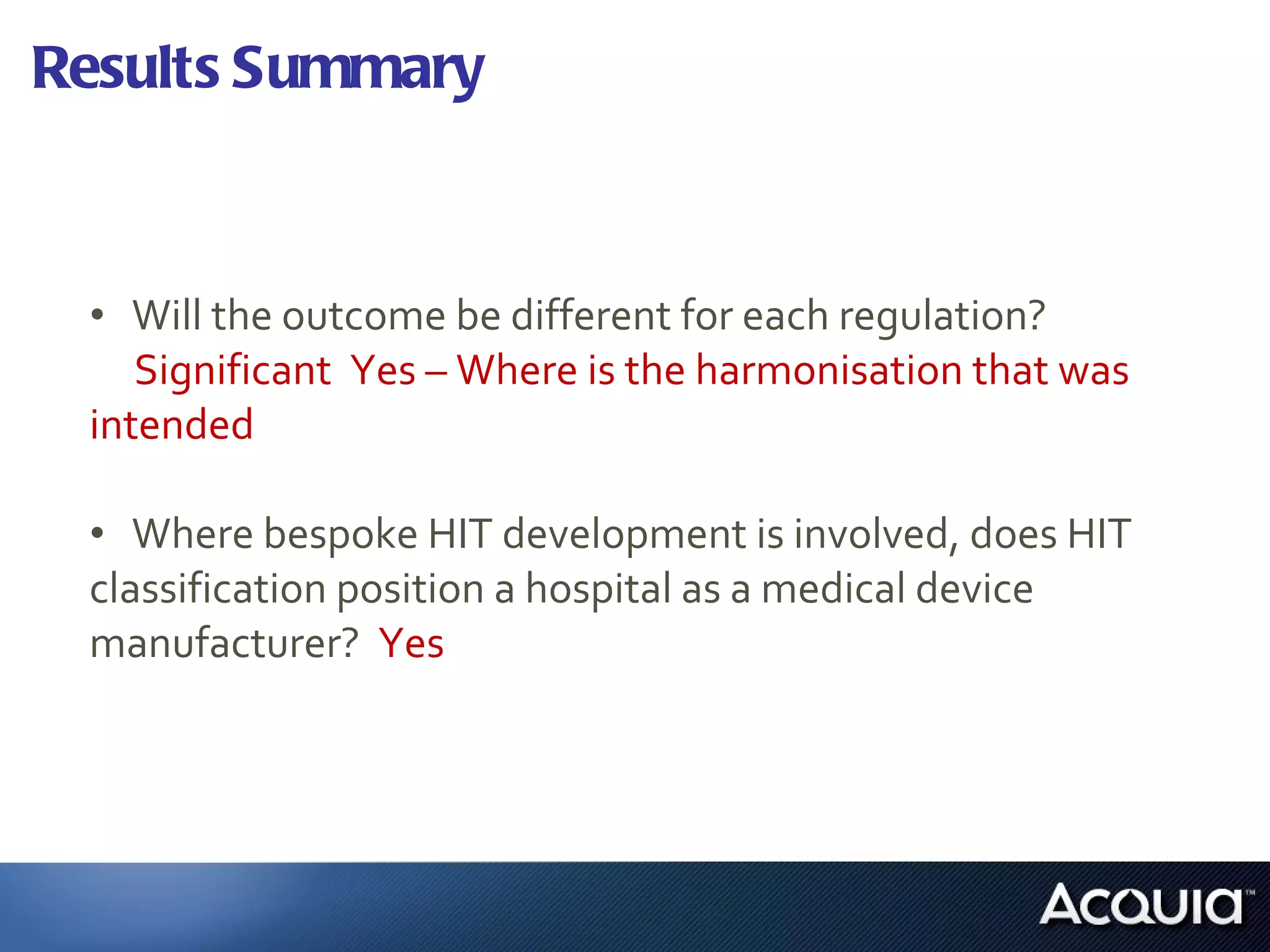
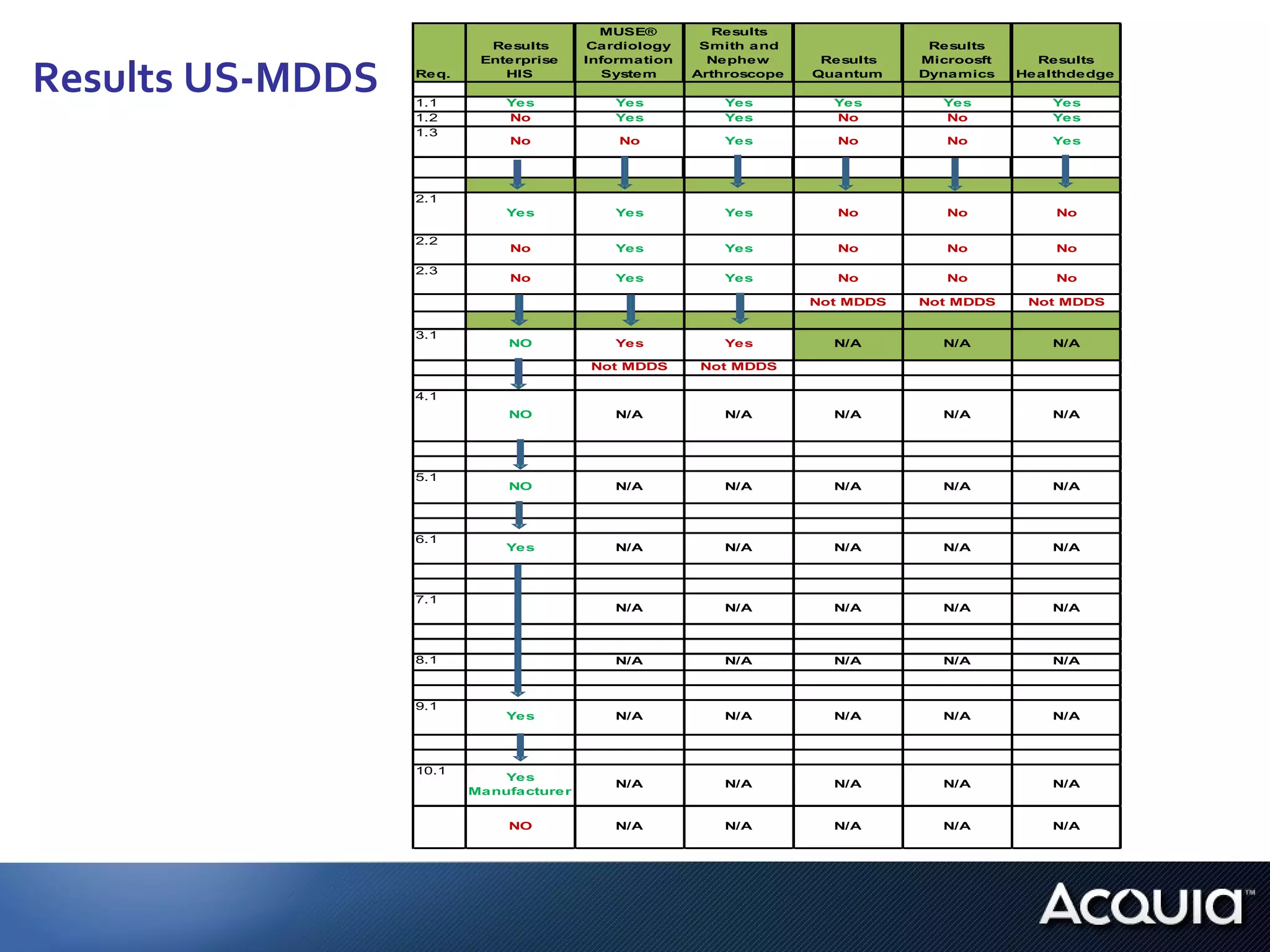
2. There are significant differences in how software would be classified under each regulation. The EU regulation considers more software to be medical devices than the US regulation.
3. If bespoke healthcare IT is developed, the hospital could be considered a medical device manufacturer under the EU regulation but not necessarily under the US regulation.
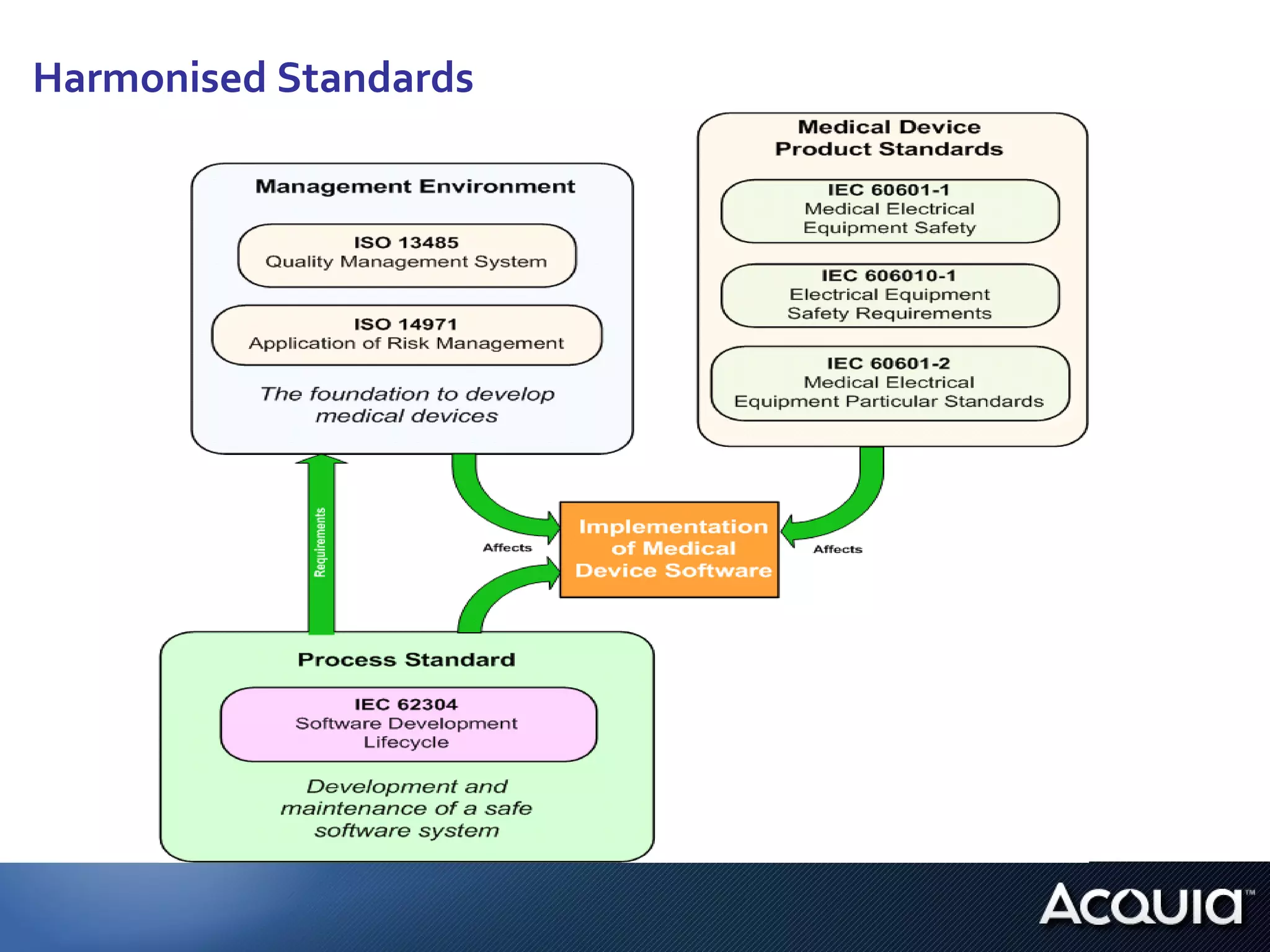
4. Further research is needed on how complementary standards and guidelines could apply, how software evaluation and vendor selection may differ between public and private institutions, and how regulations apply to software in general practitioner offices.










![Thank you Email: [email_address] frank.maxwell@gmail.com Linkedin: http://ie.linkedin.com/in/frankmaxwell](https://image.slidesharecdn.com/softwareasdevicefrankmaxwell-111128061801-phpapp02/75/Software-As-Device-Frank-Maxwell-23-2048.jpg)