Embed presentation
Downloaded 287 times















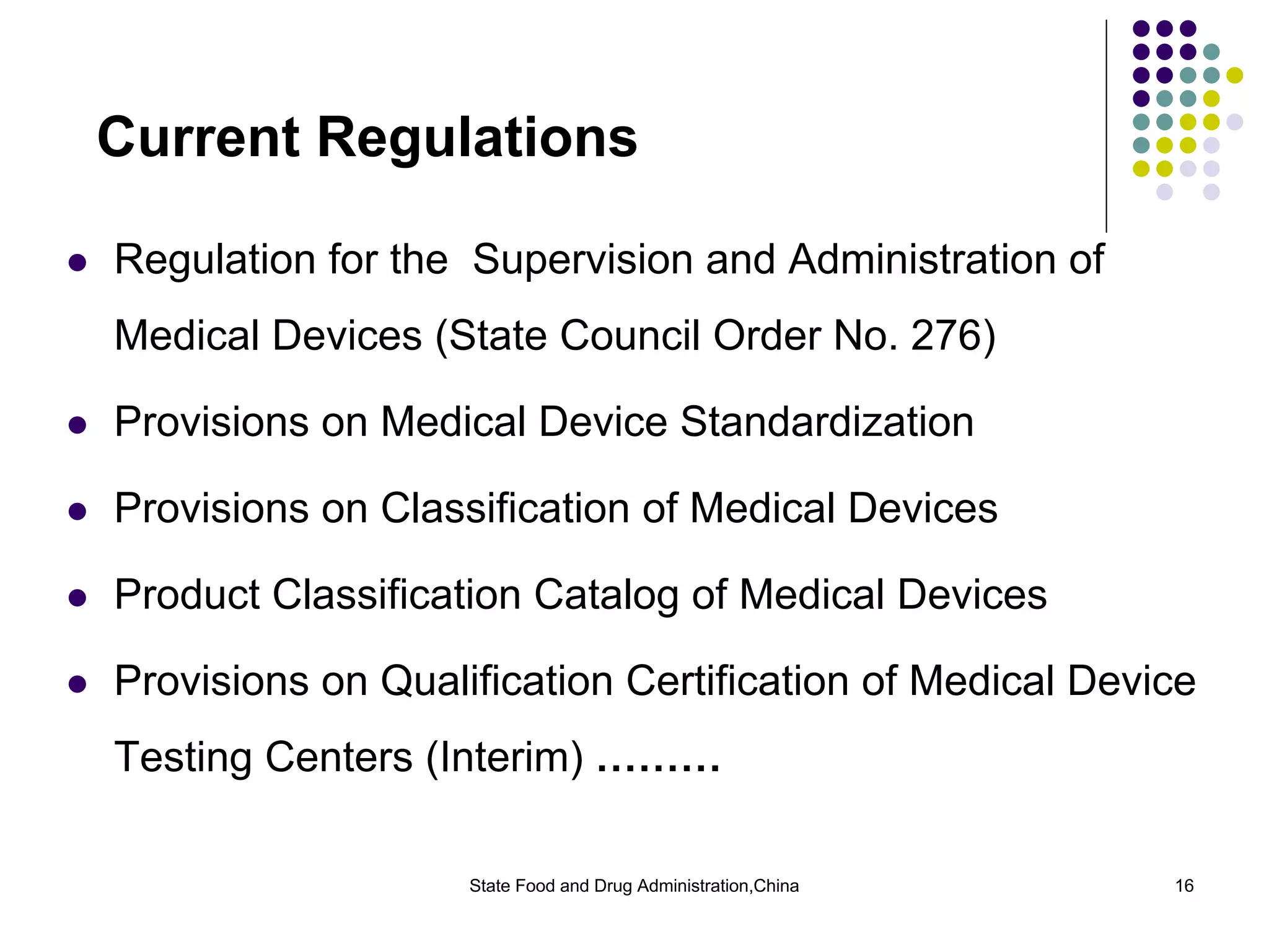



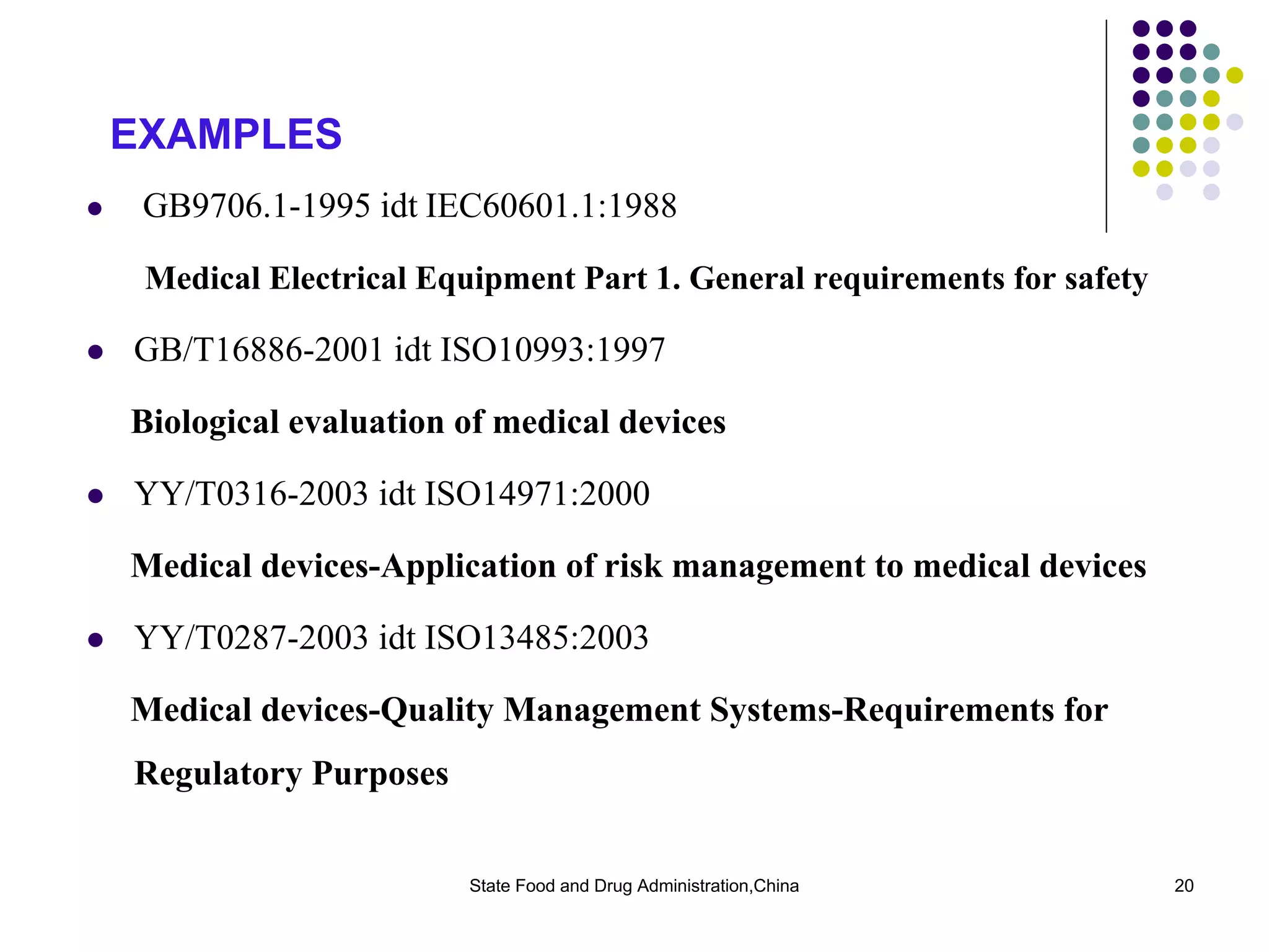
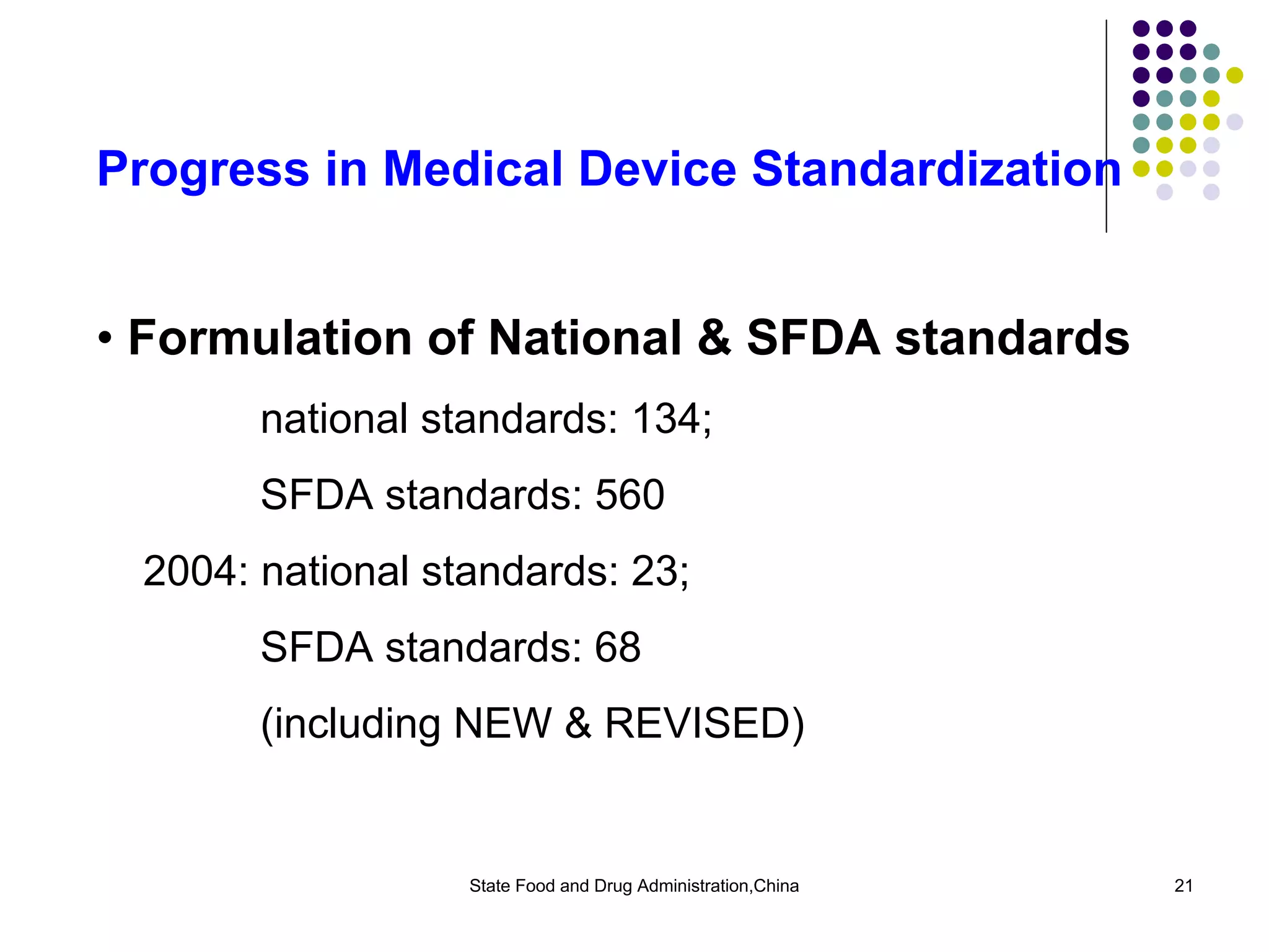













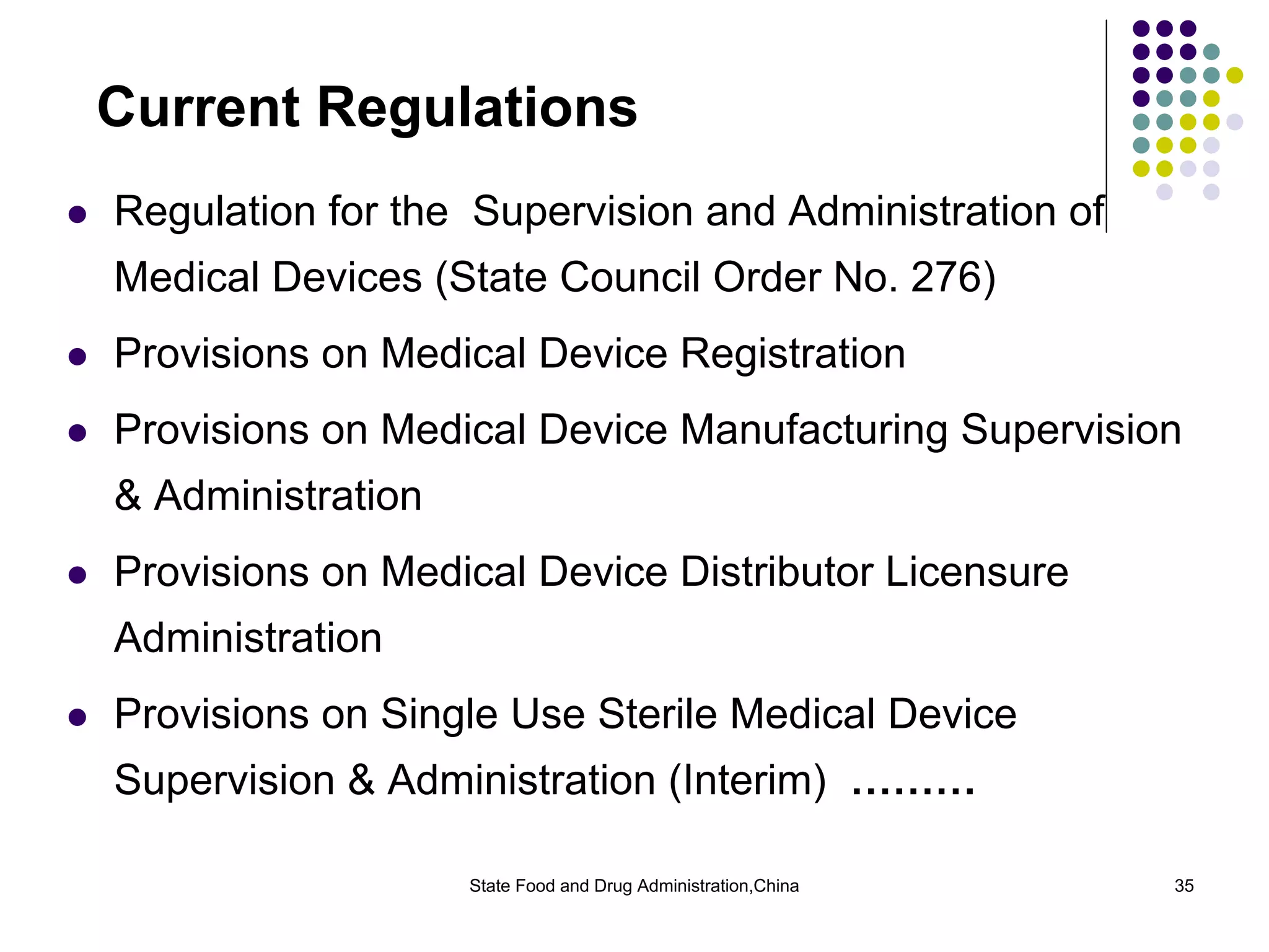




















The document outlines the regulatory framework for medical devices in China, governed by the State Food and Drug Administration (SFDA) established in 2003. It details the administrative organizations, regulations, classification of medical devices, standardization efforts, and manufacturing supervision processes. Additionally, it discusses medical device vigilance, including adverse event monitoring and licensing requirements for manufacturers.






















































