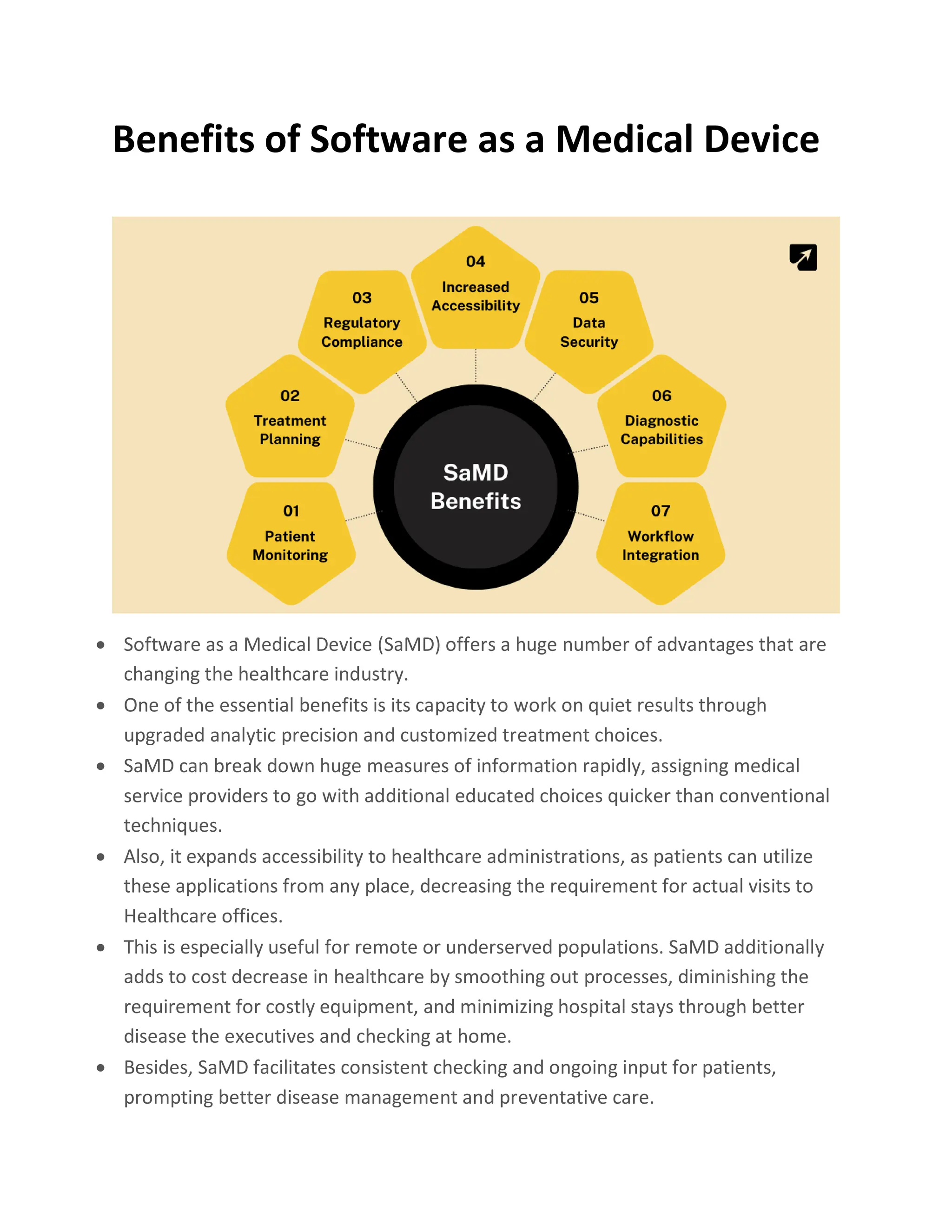
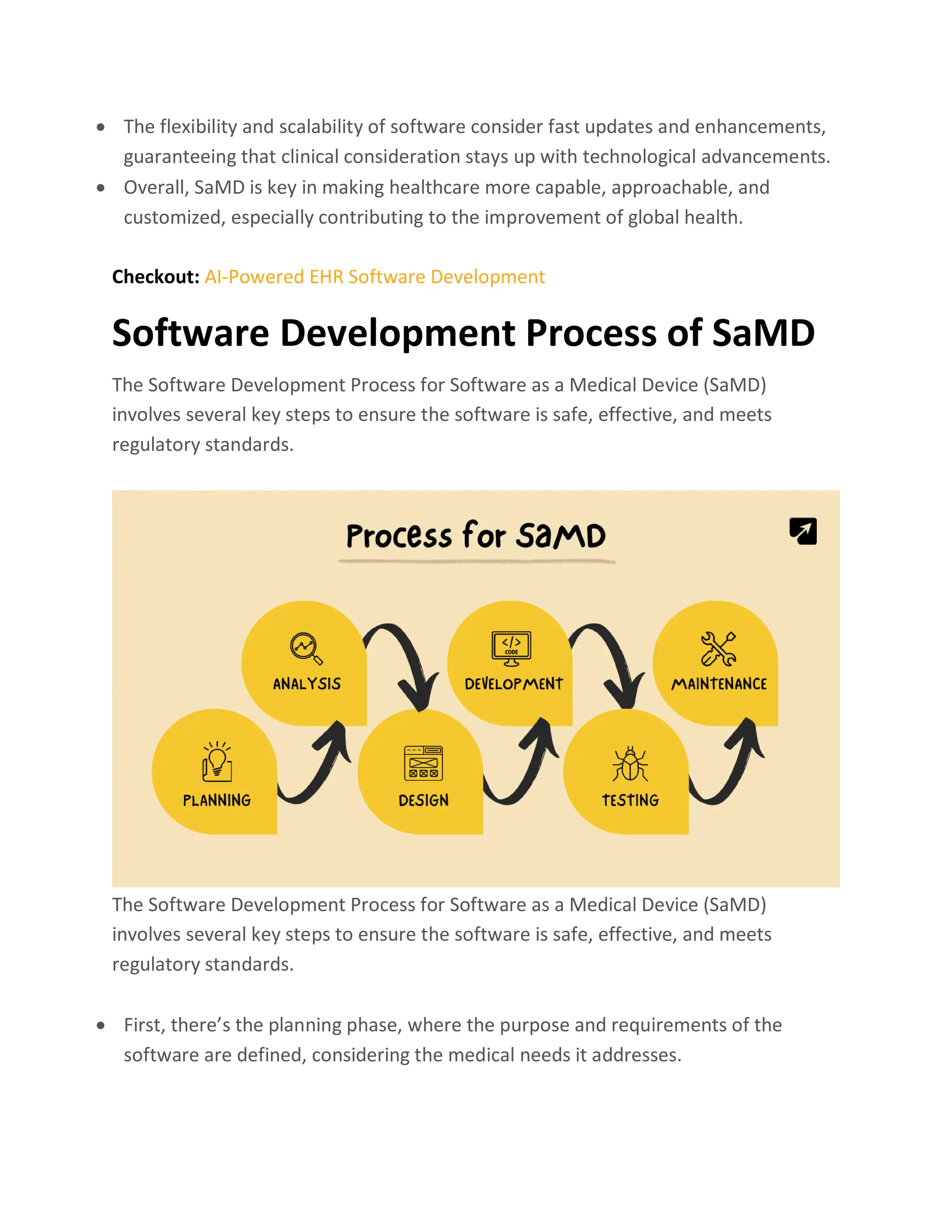
Software as a Medical Device (SaMD) represents a groundbreaking innovation using software to perform medical functions without the need for traditional devices, enhancing healthcare precision and personalization. SaMD is regulated differently worldwide, ensuring safety and efficacy, with models demonstrating its benefits including improved patient outcomes, accessibility, and cost reduction. The development process follows strict guidelines from planning to deployment, emphasizing collaboration and compliance to facilitate effective healthcare solutions.