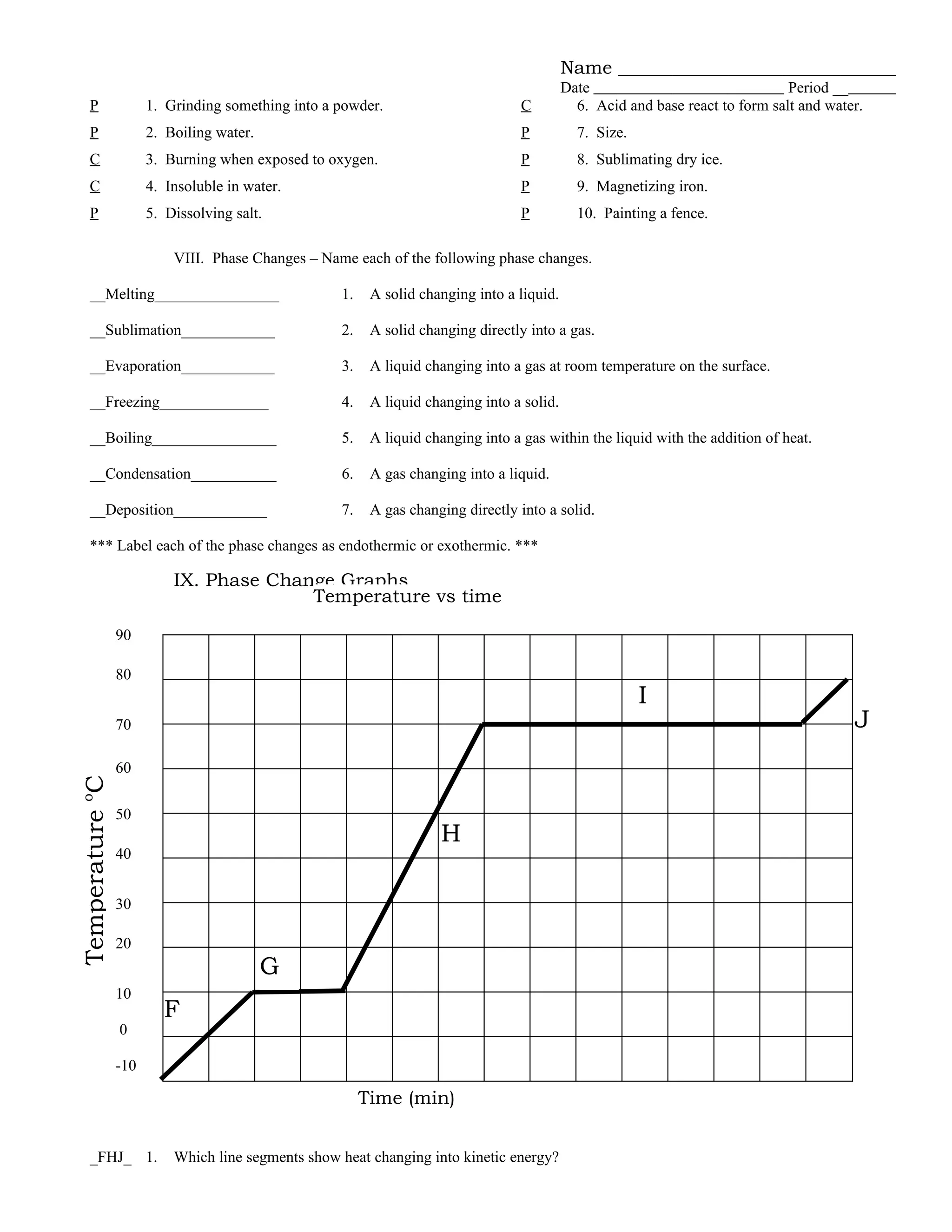
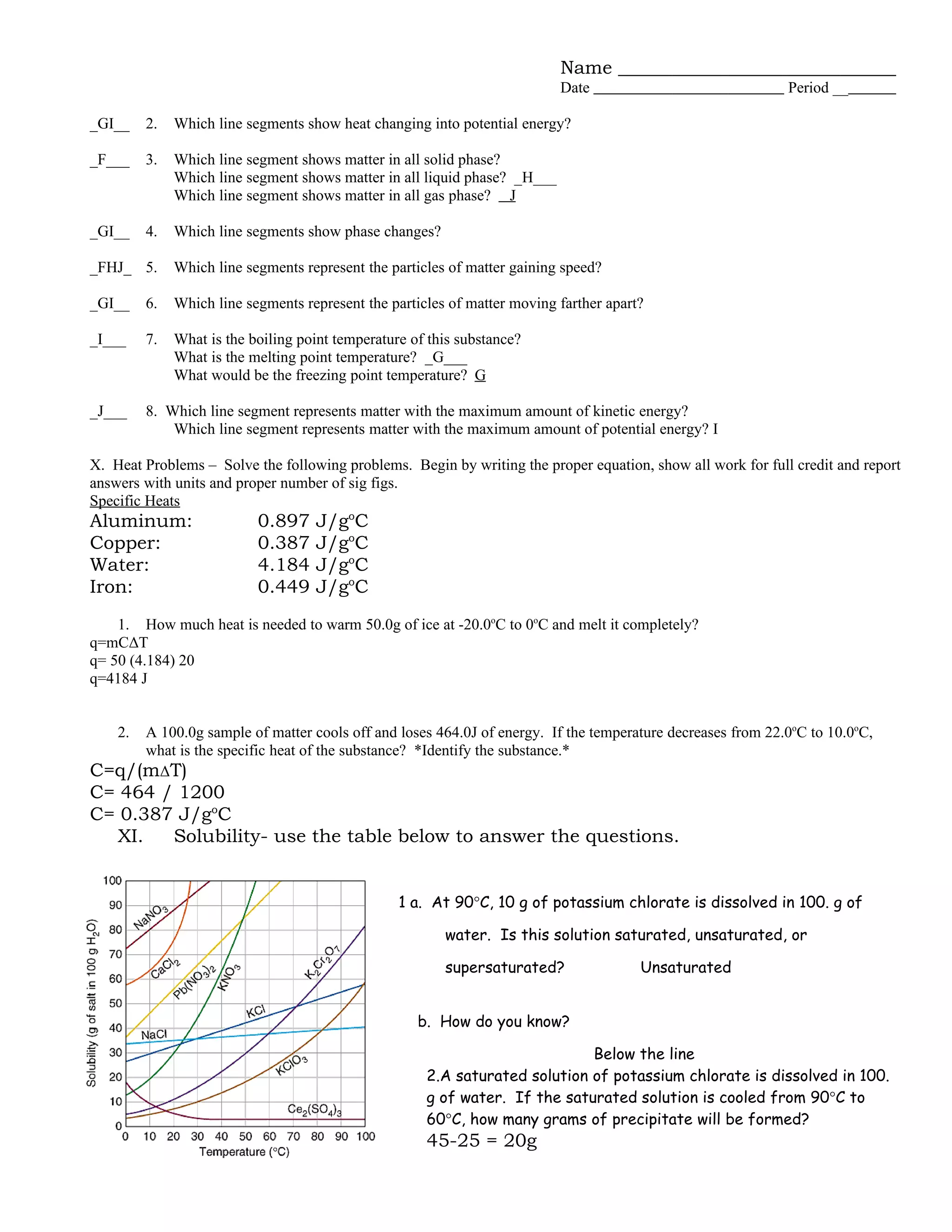
The document provides a review of chemistry concepts related to matter and energy, including defining key terms like physical and chemical properties, classifying mixtures and phases of matter, explaining phase changes and energy transfers. It covers the particle model of matter and kinetic molecular theory, properties of solutions, and classifying substances and changes as physical or chemical. Sample problems are included on heat transfer during phase changes and calculating energy changes.