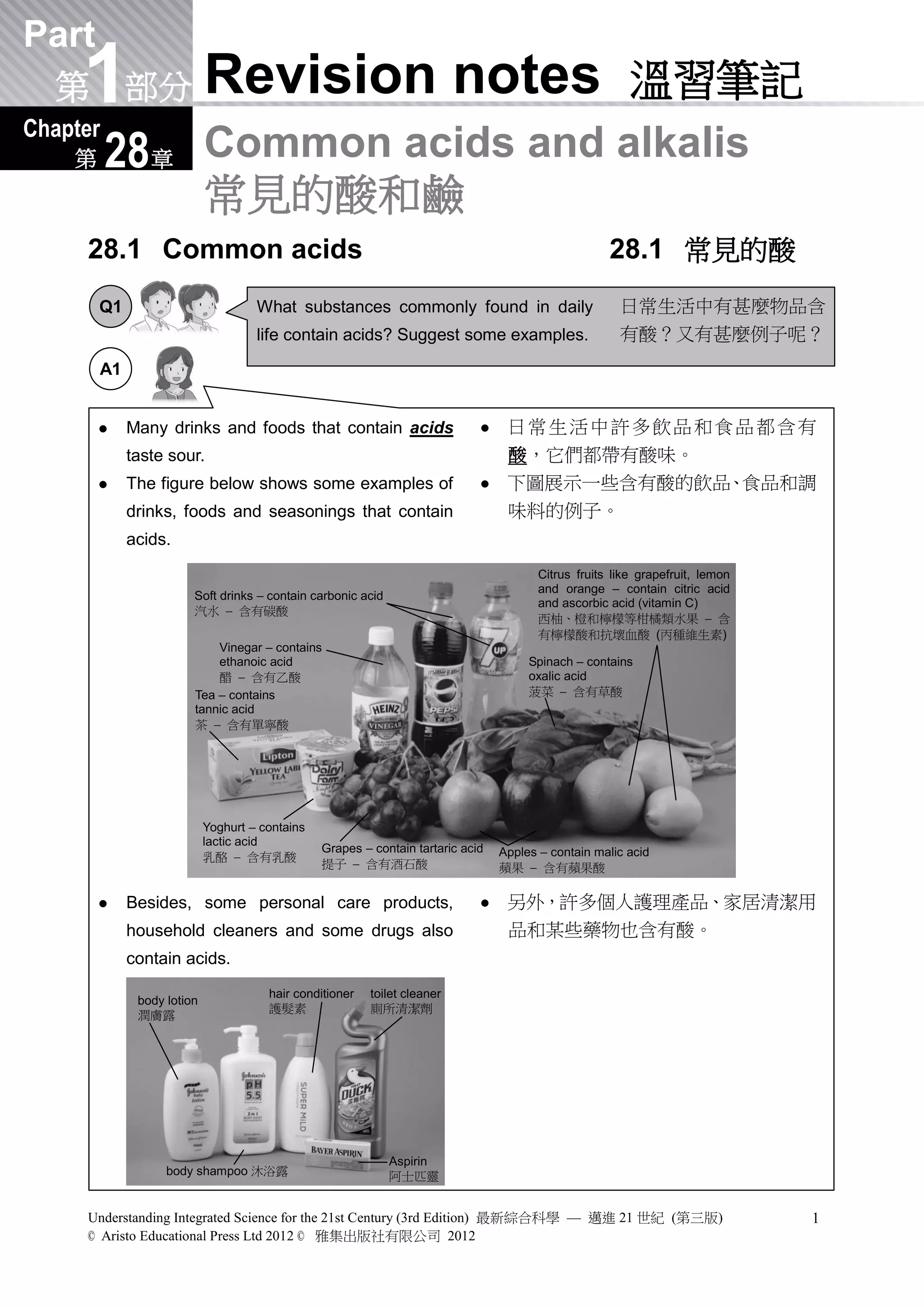
1) The document discusses common acids and alkalis found in daily life and laboratories. It provides examples of acids in drinks, foods and household products. Common acids used in laboratories include hydrochloric acid, sulfuric acid and nitric acid.
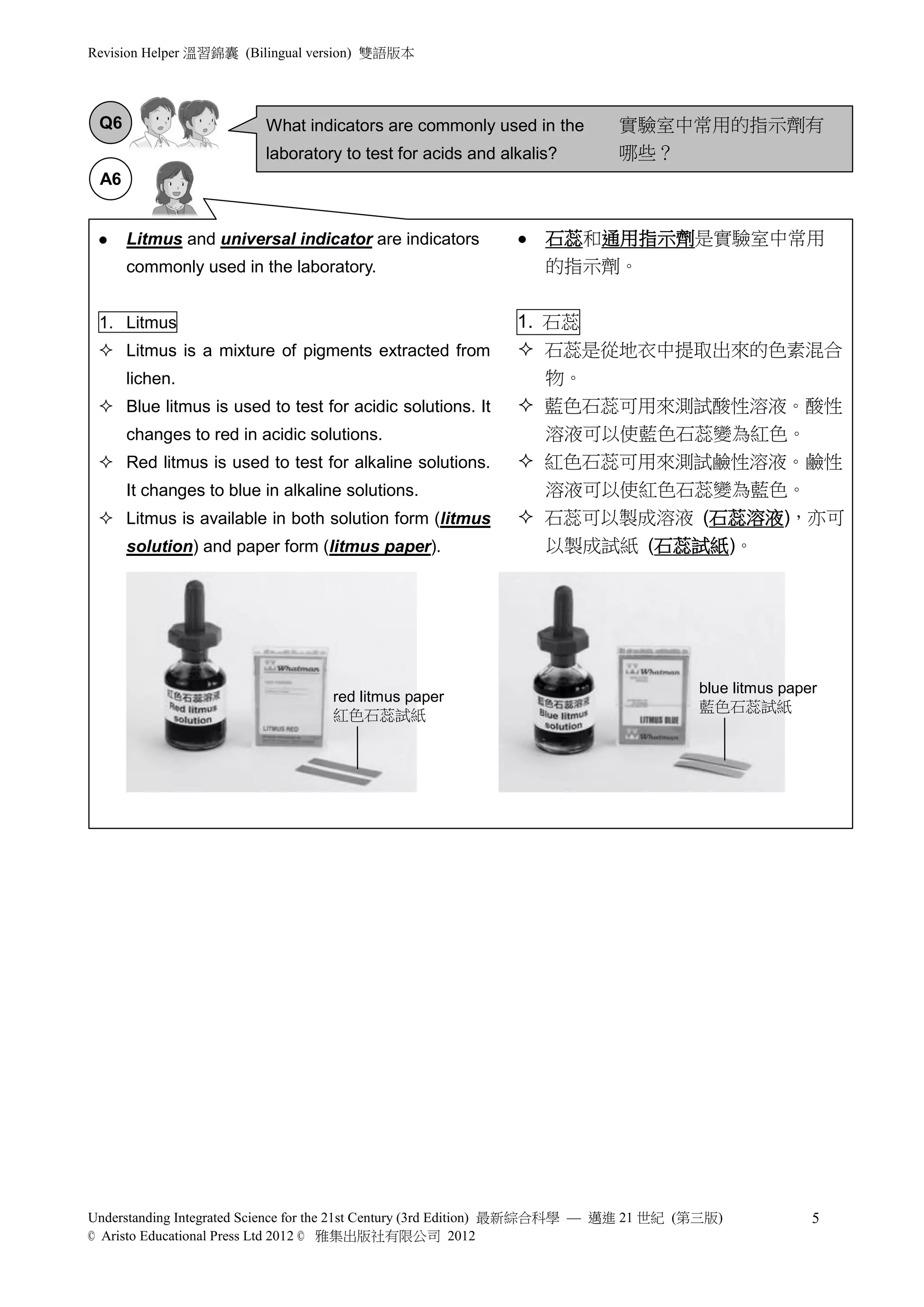
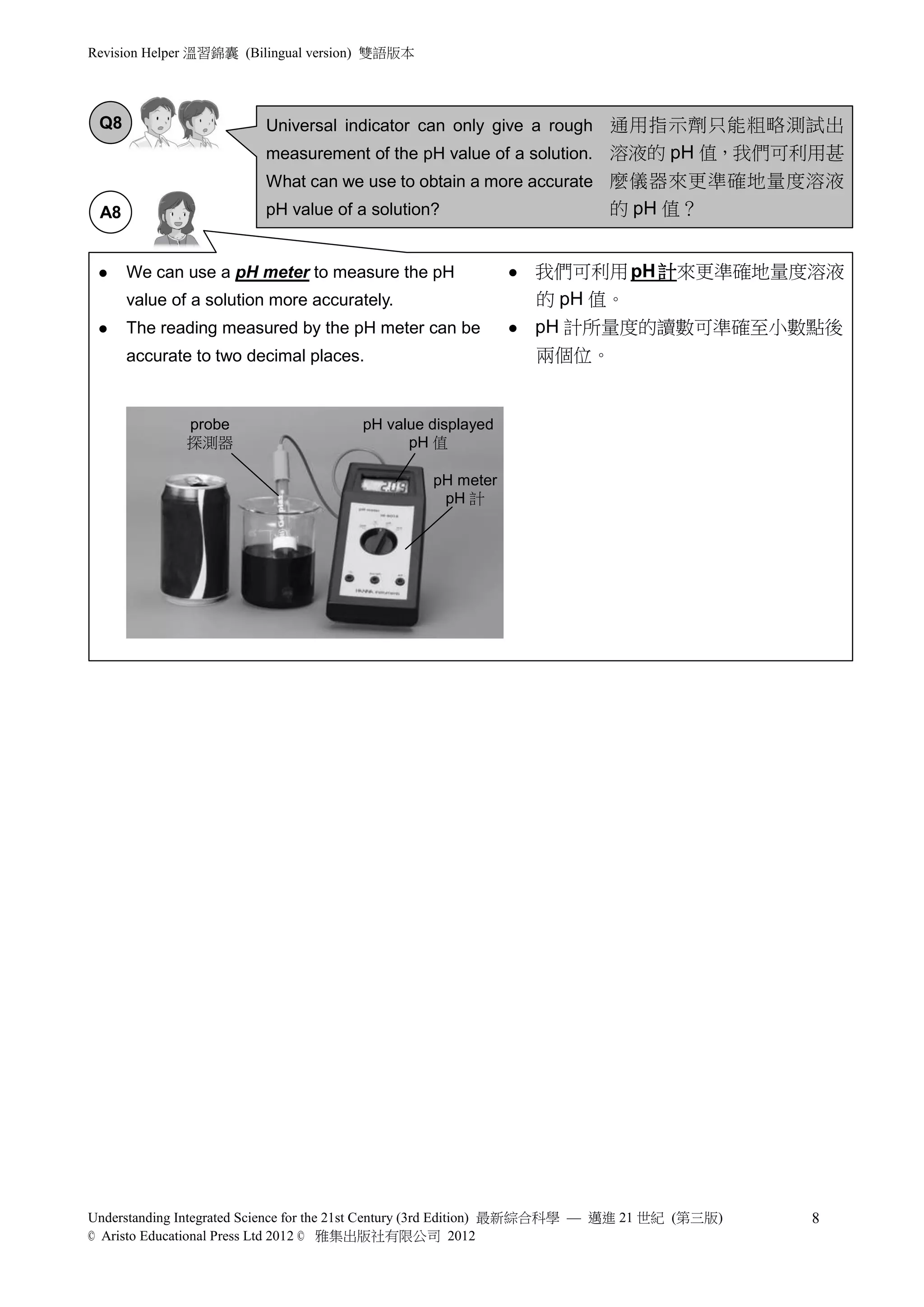
2) Natural plant pigments and litmus are discussed as indicators for testing acids and alkalis. Litmus paper or solution changes color between red and blue depending on whether the solution is acidic or alkaline.
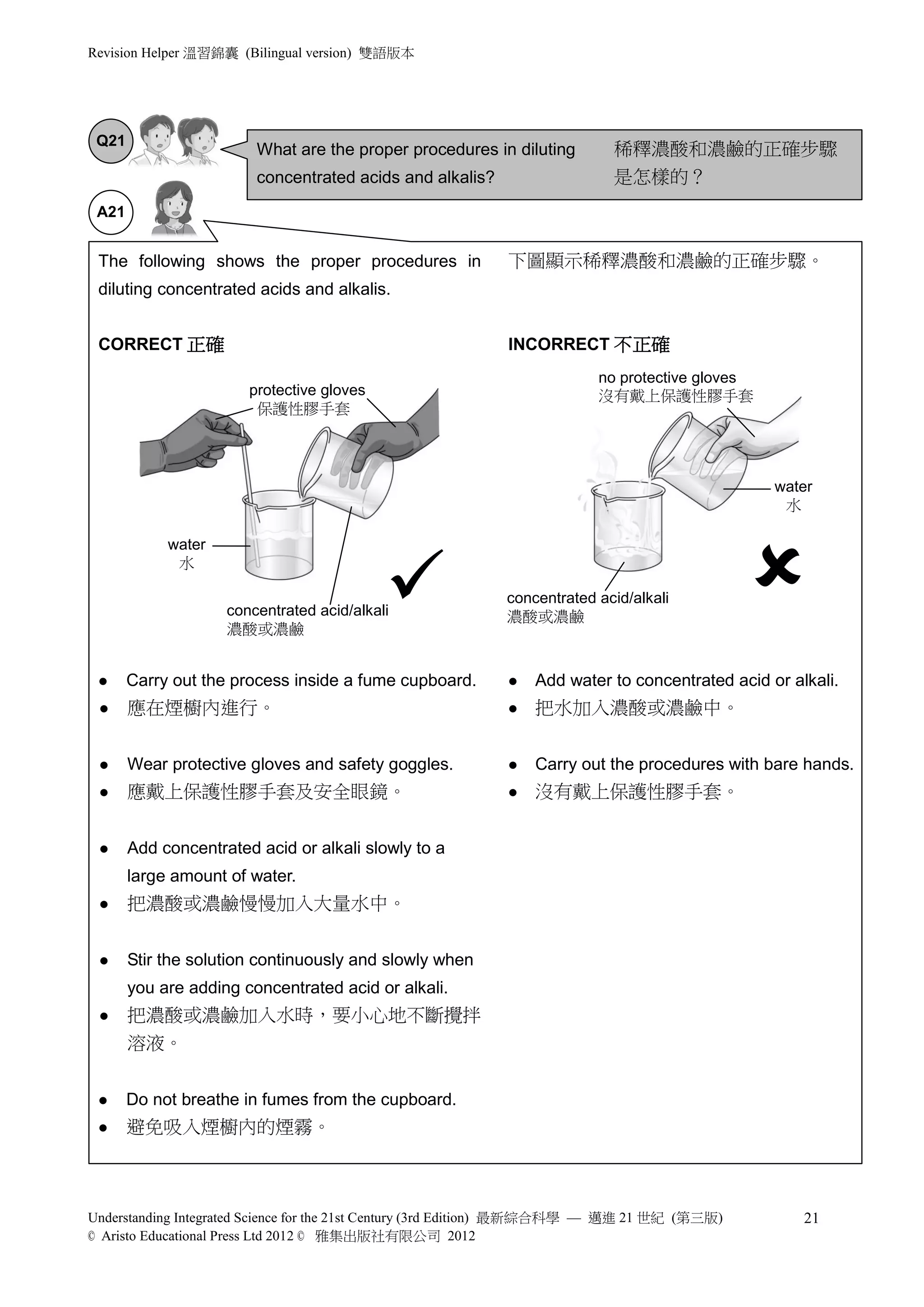
3) Safety precautions are emphasized when handling acids and alkalis due to their corrosive properties. Personal protective equipment should be worn and direct contact avoided.