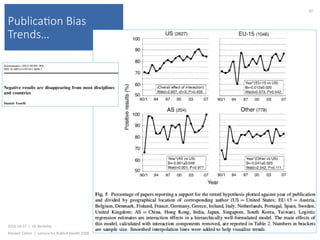
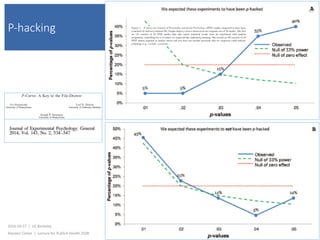
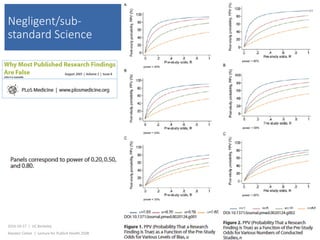
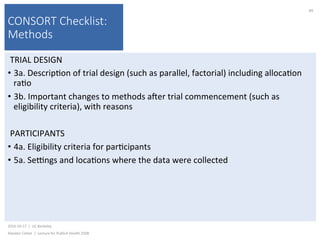
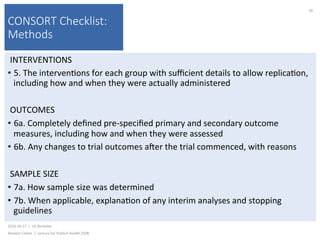
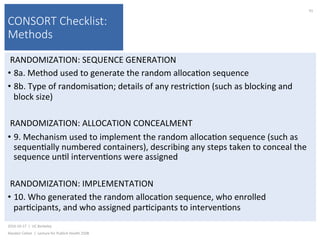
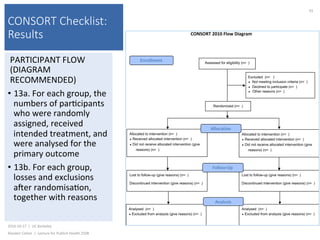
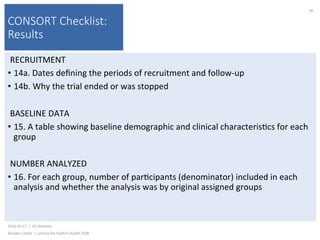
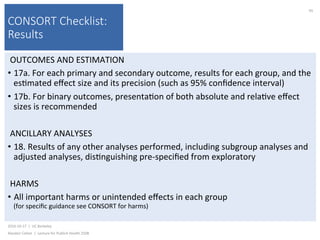
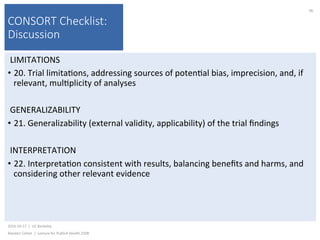
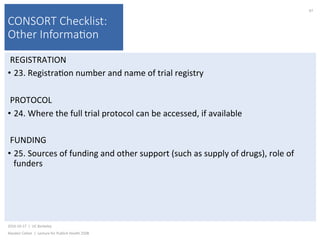
This document contains slides from a lecture on public health given by Alasdair Cohen at UC Berkeley on October 17, 2016. The slides discuss various topics related to research quality including publication bias, p-hacking, negligent science, Cochrane reviews, transparent reporting, and the CONSORT checklist. The CONSORT checklist is explained in detail over several slides, outlining the key elements that should be reported in randomized controlled trials to improve transparency and replication.