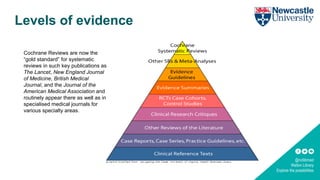
The Cochrane Library is a collection of databases that provides high-quality, systematic reviews of the effects of healthcare interventions. It contains the Cochrane Database of Systematic Reviews, which summarizes conclusions about the effectiveness of interventions based on empirical evidence. Cochrane Reviews are considered the gold standard for systematic reviews because they are conducted to the highest methodological quality and updated regularly. The Cochrane Library also includes databases of controlled trials, reviews of other systematic reviews, methodology research, and economic evaluations to inform evidence-based healthcare decisions.