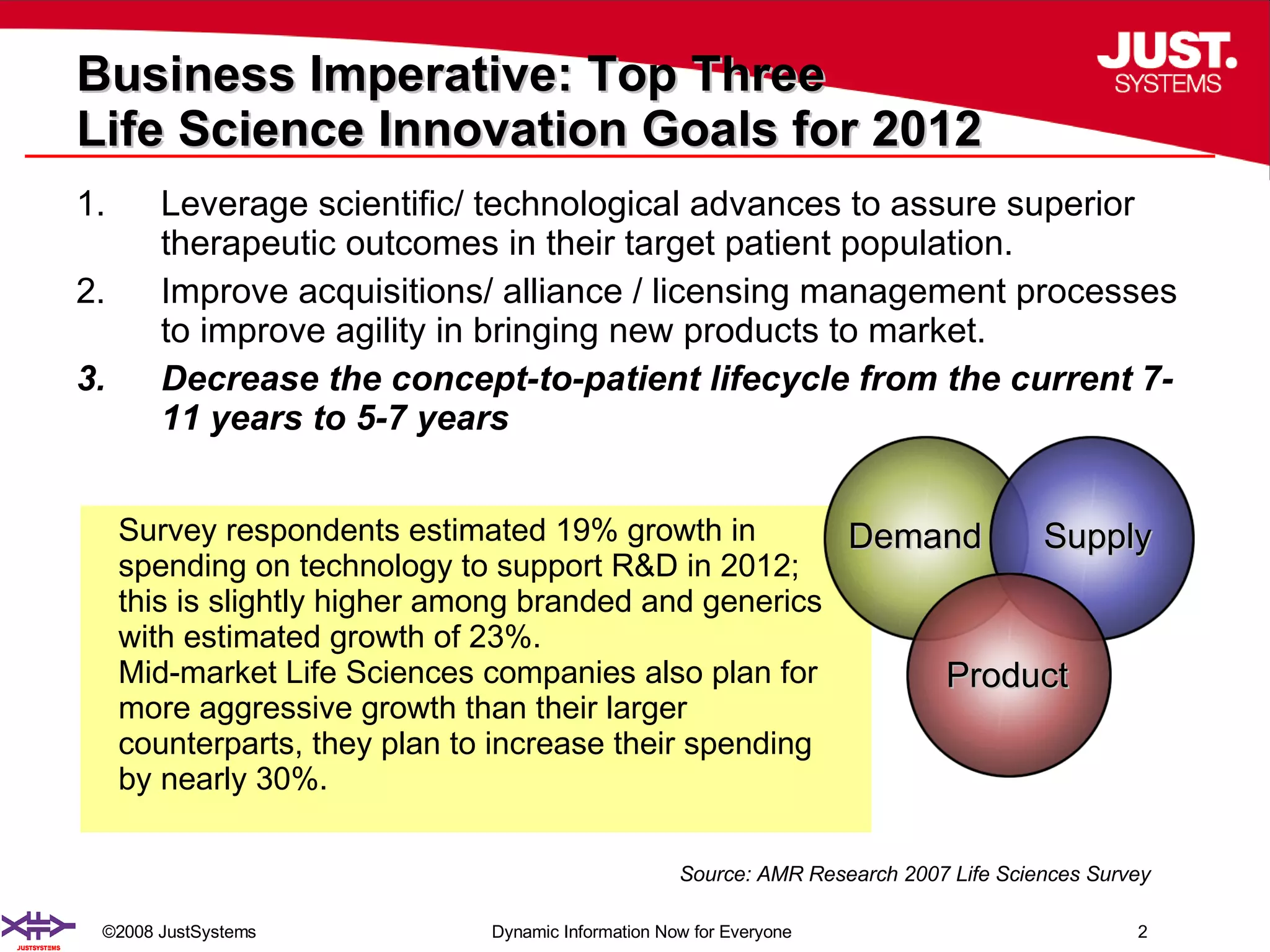
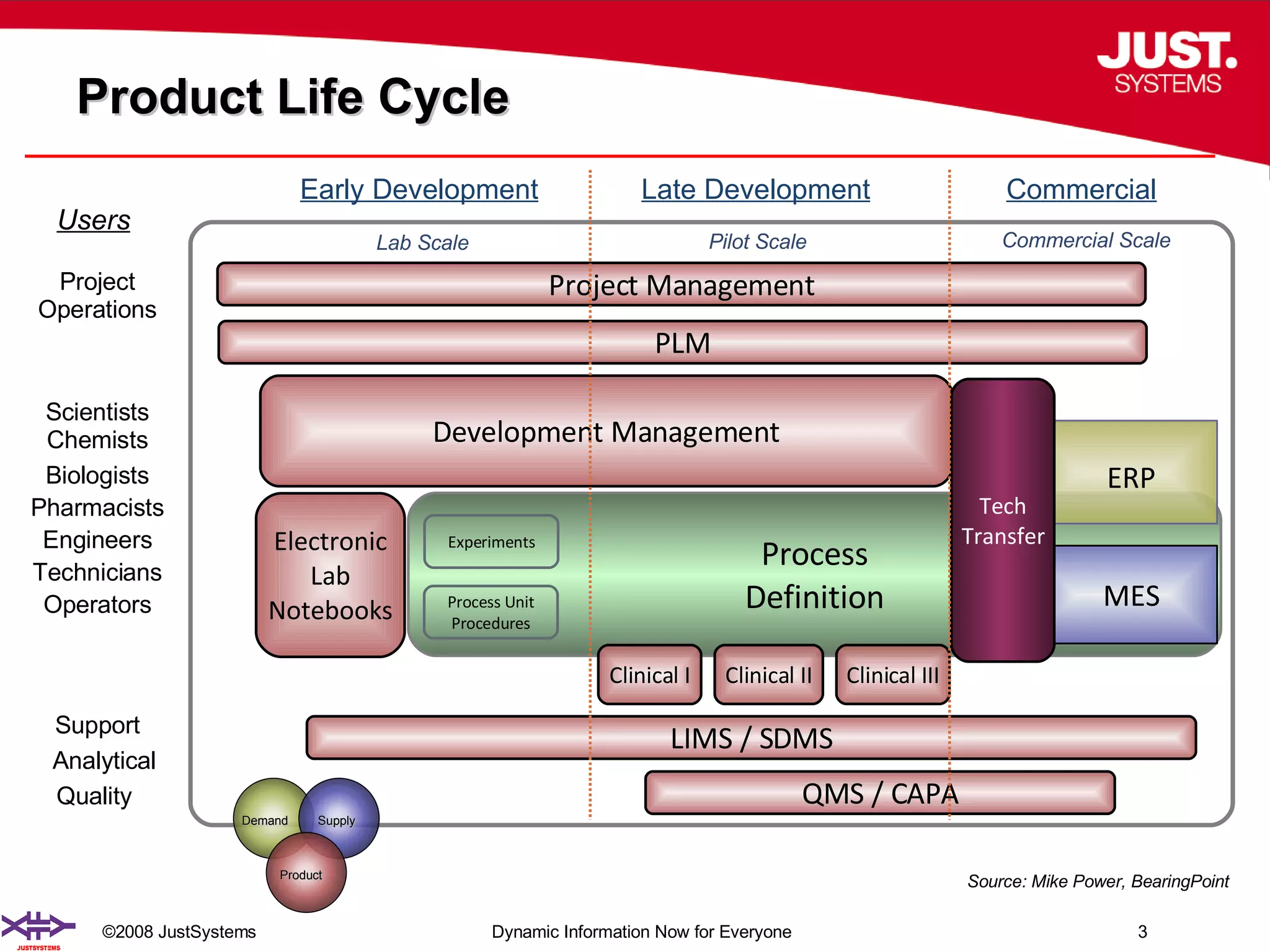
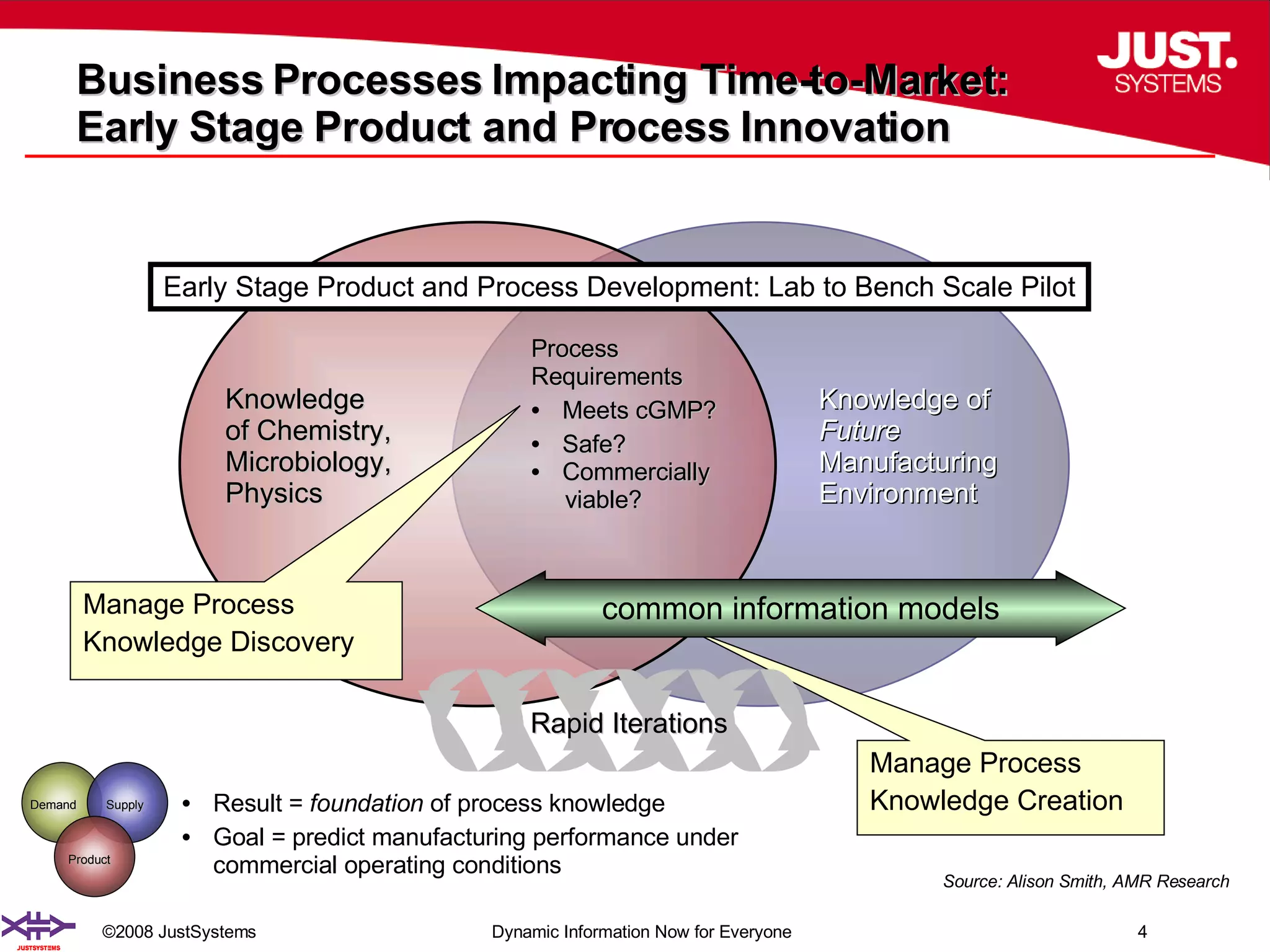
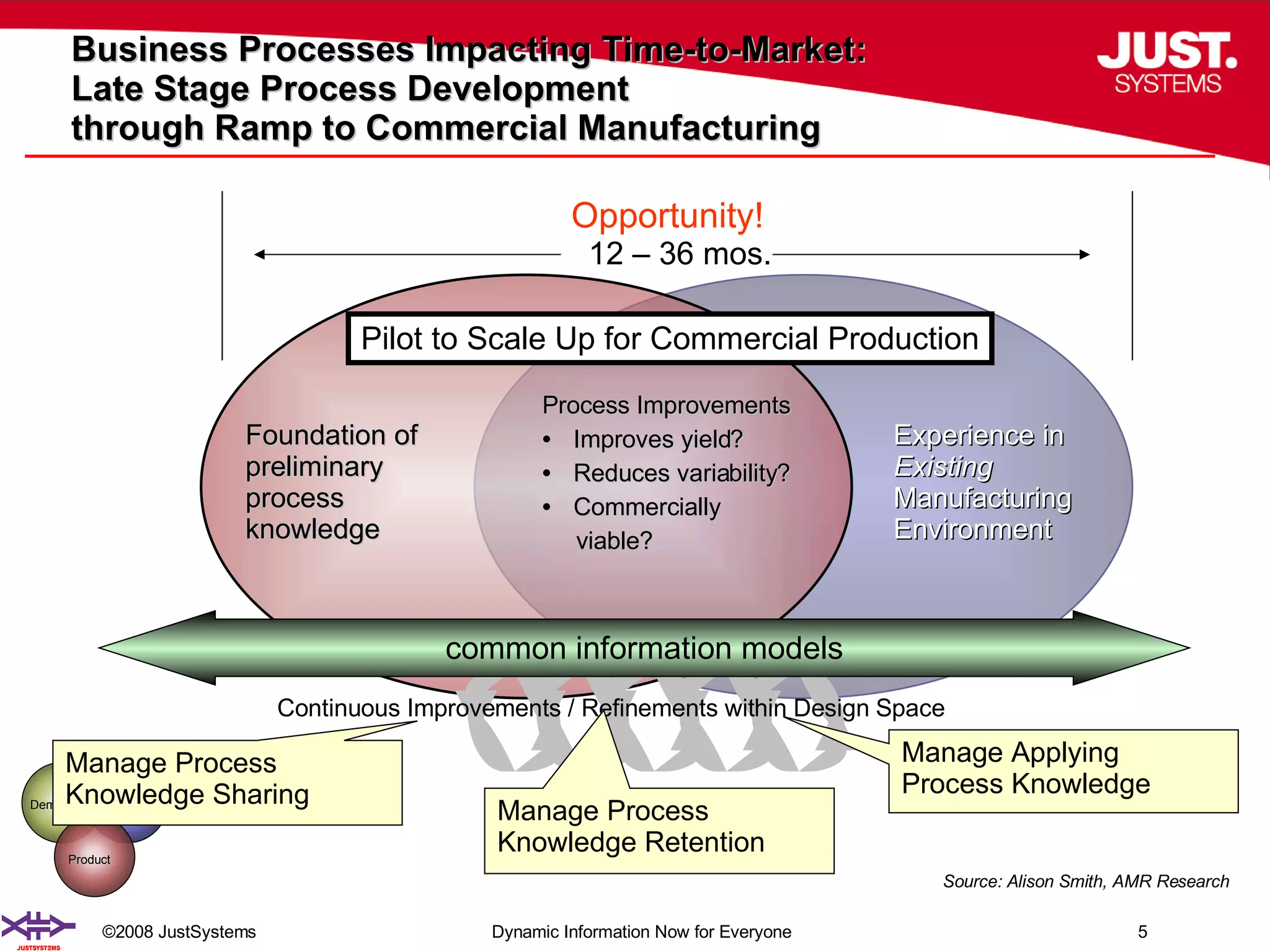
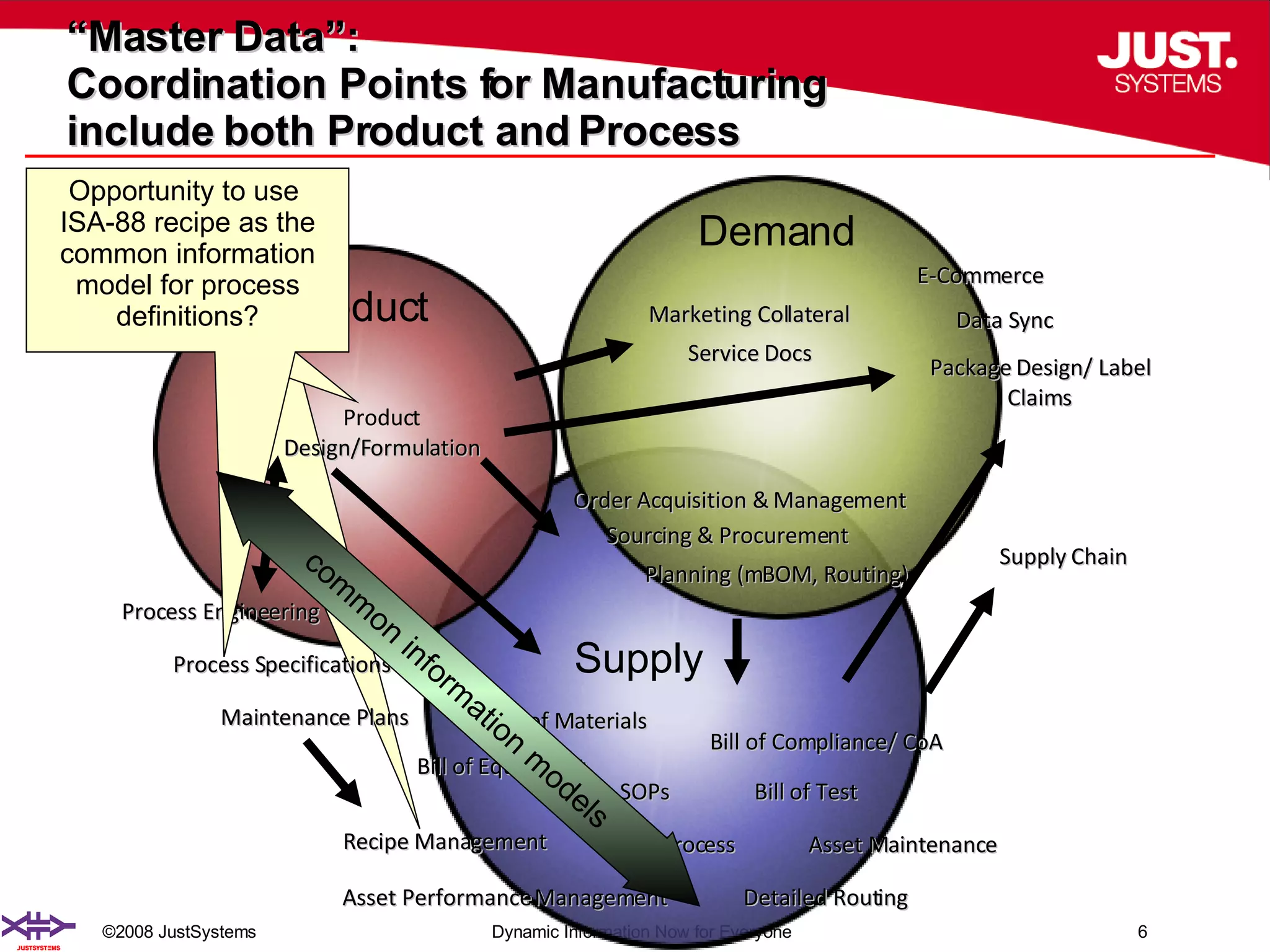
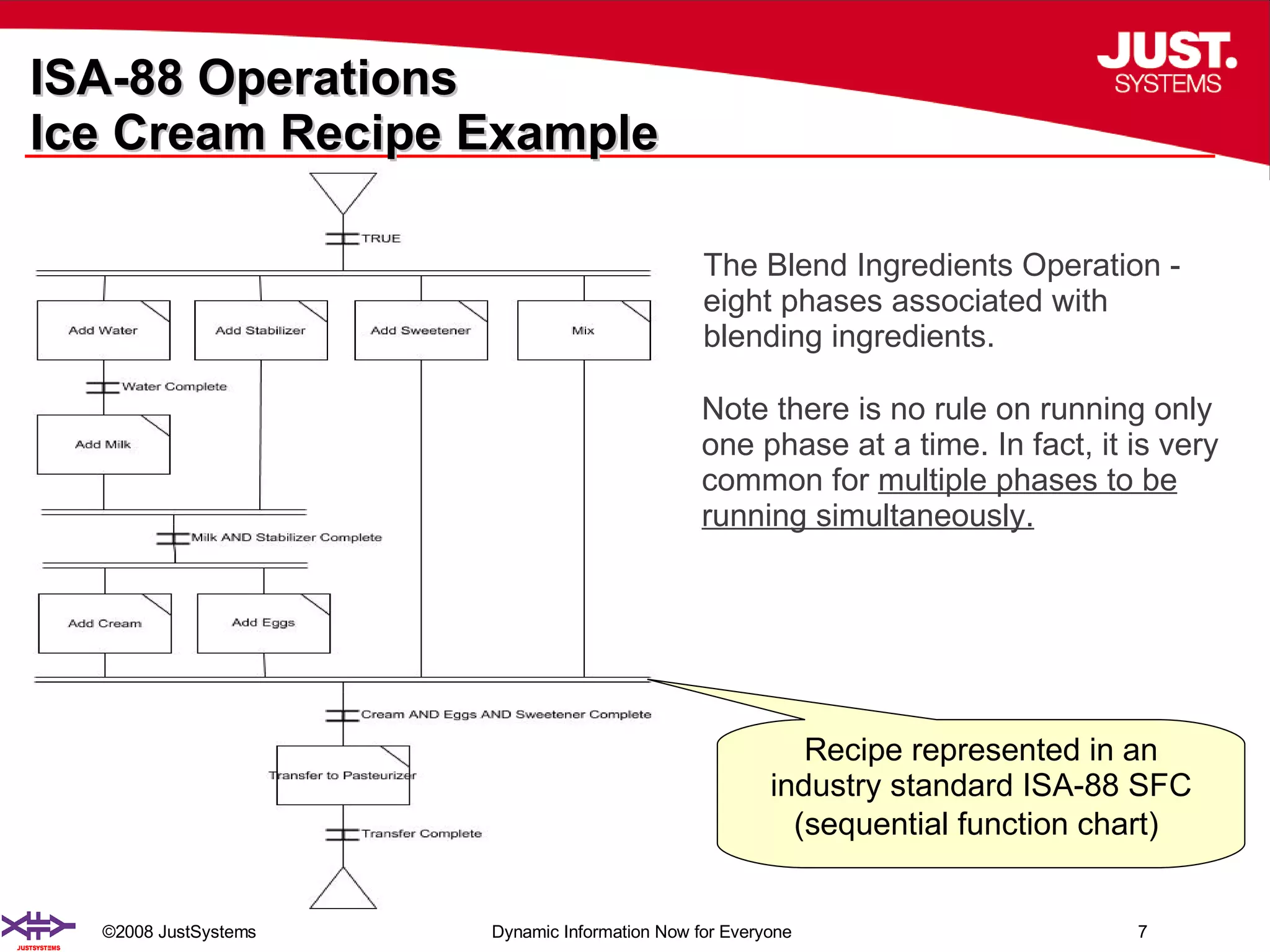
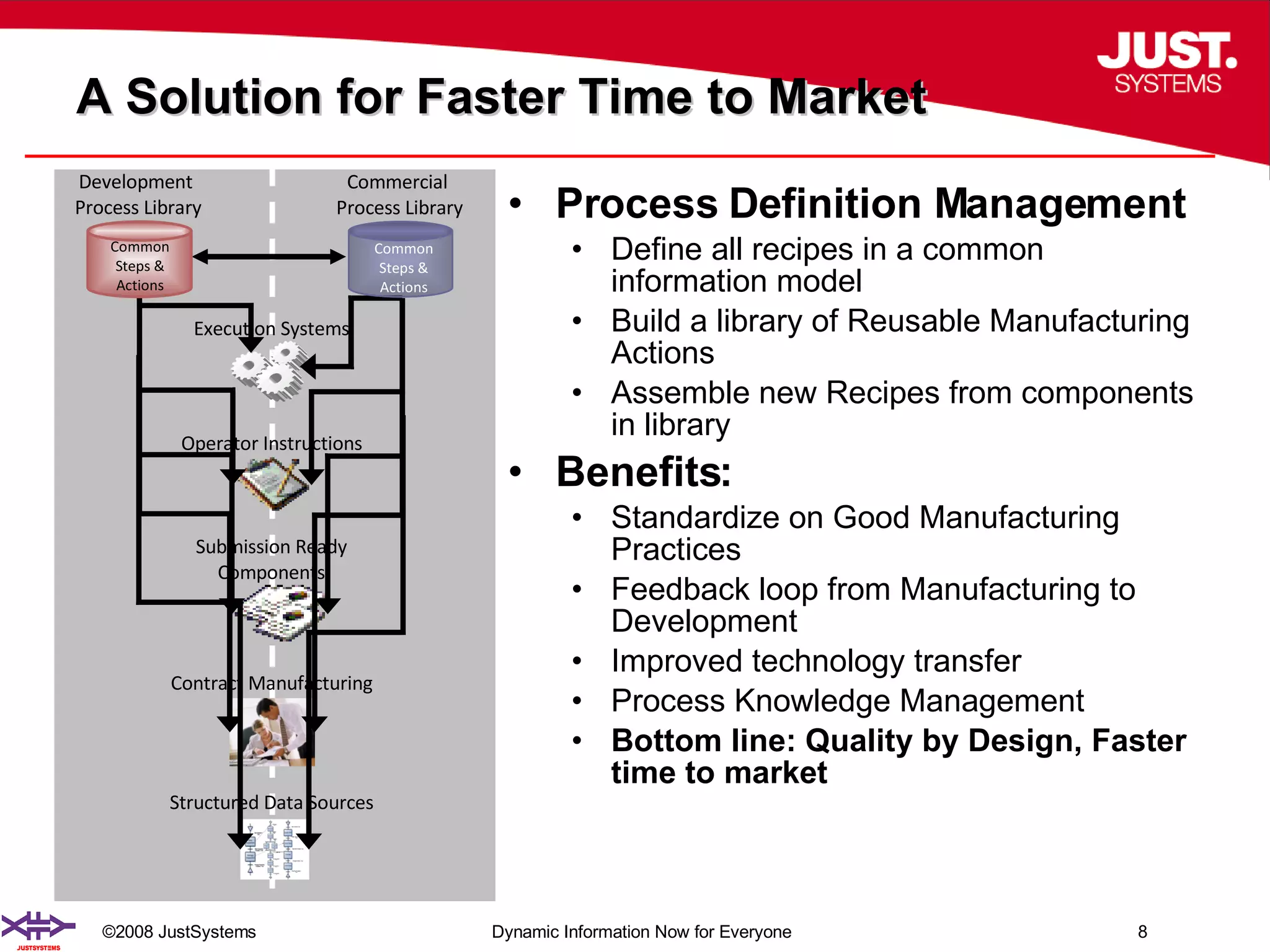
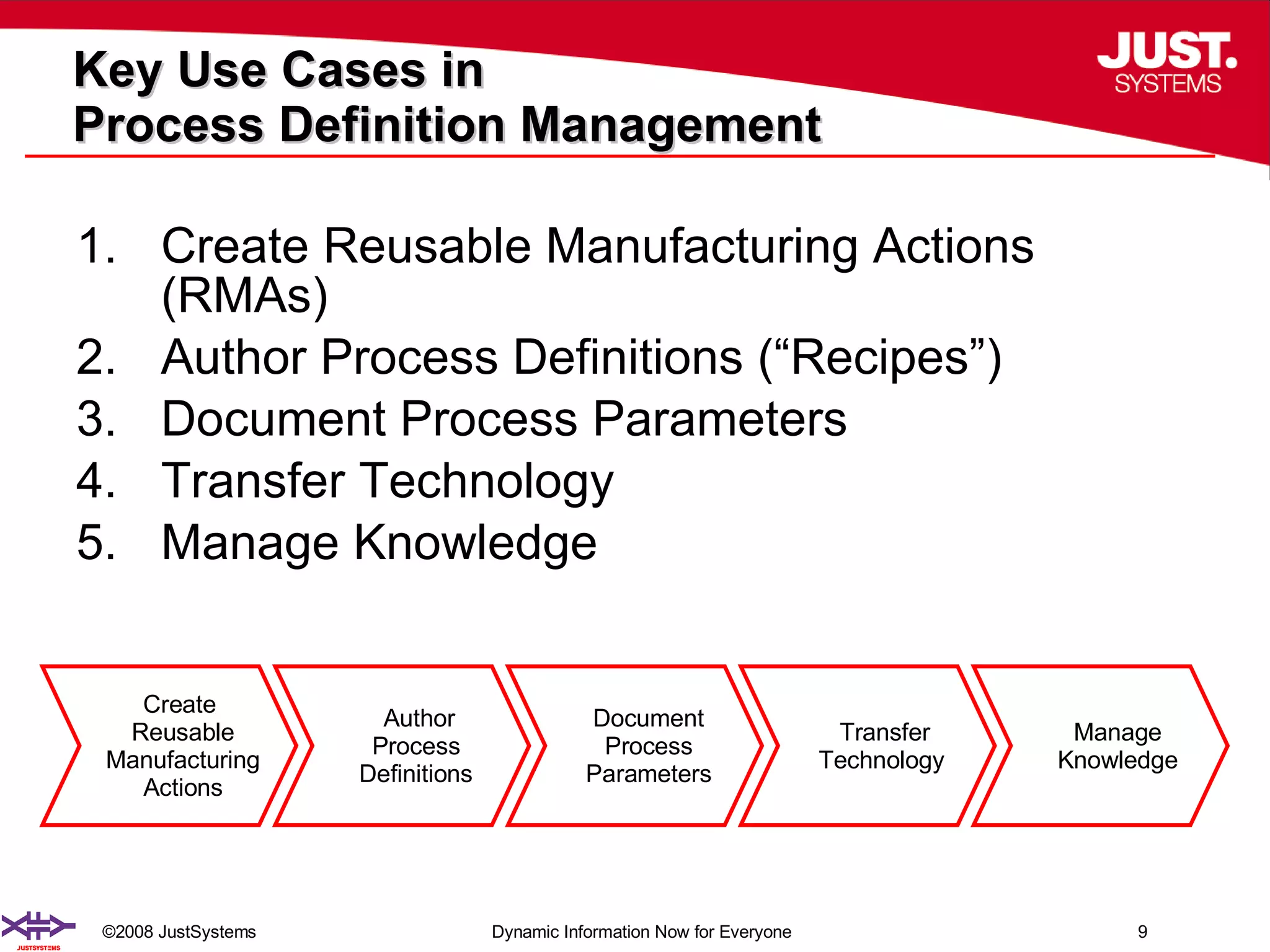
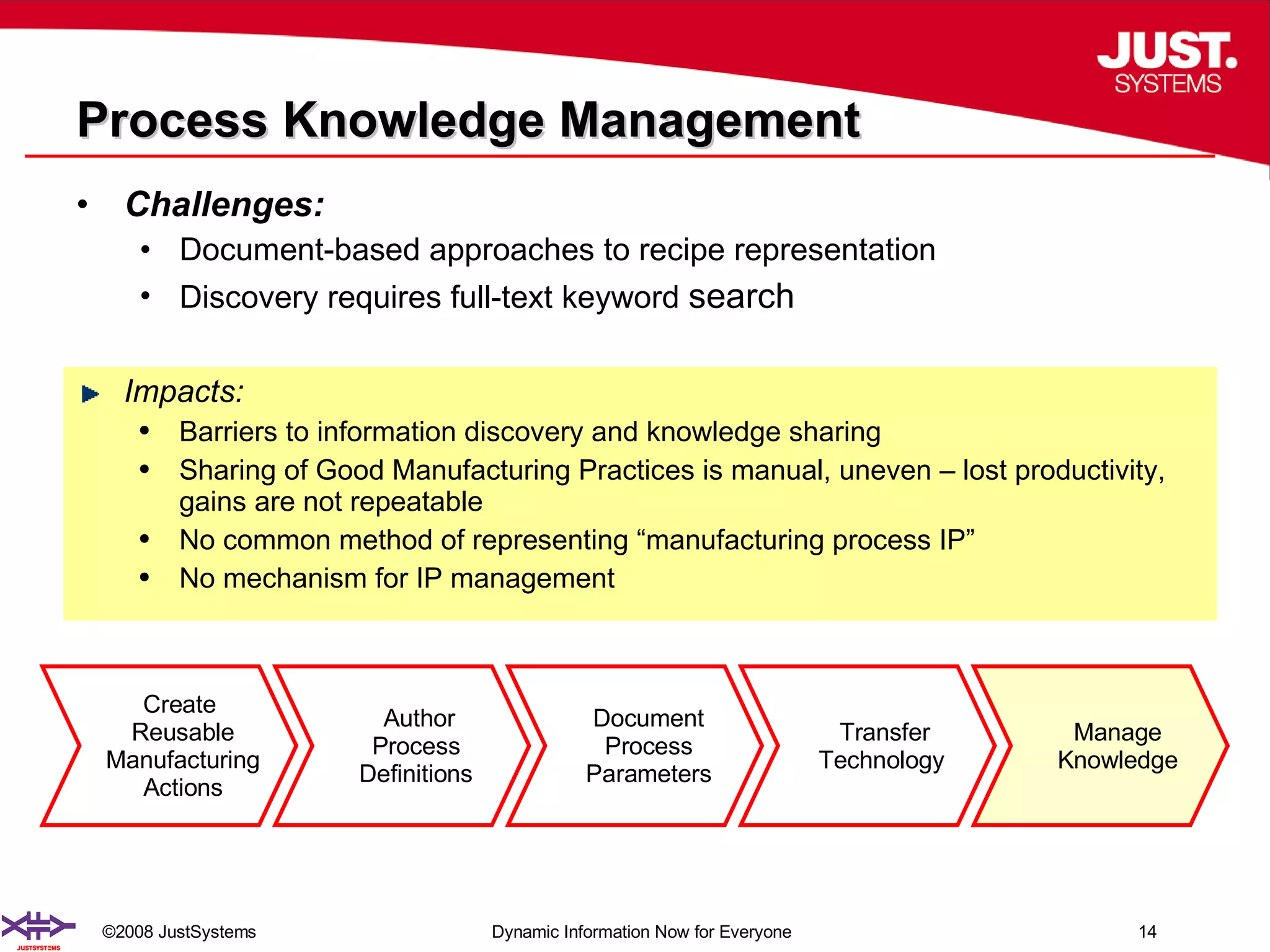
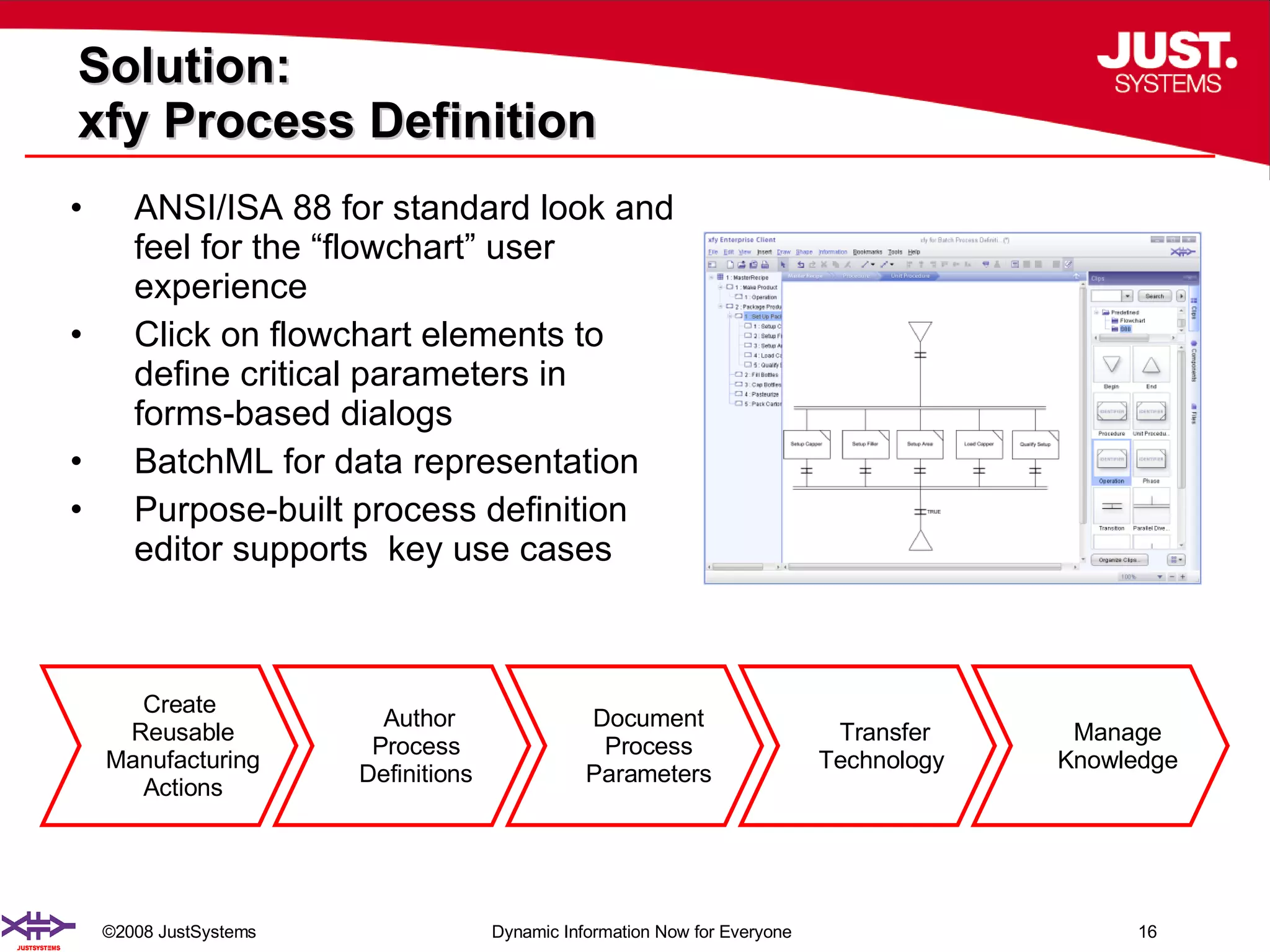
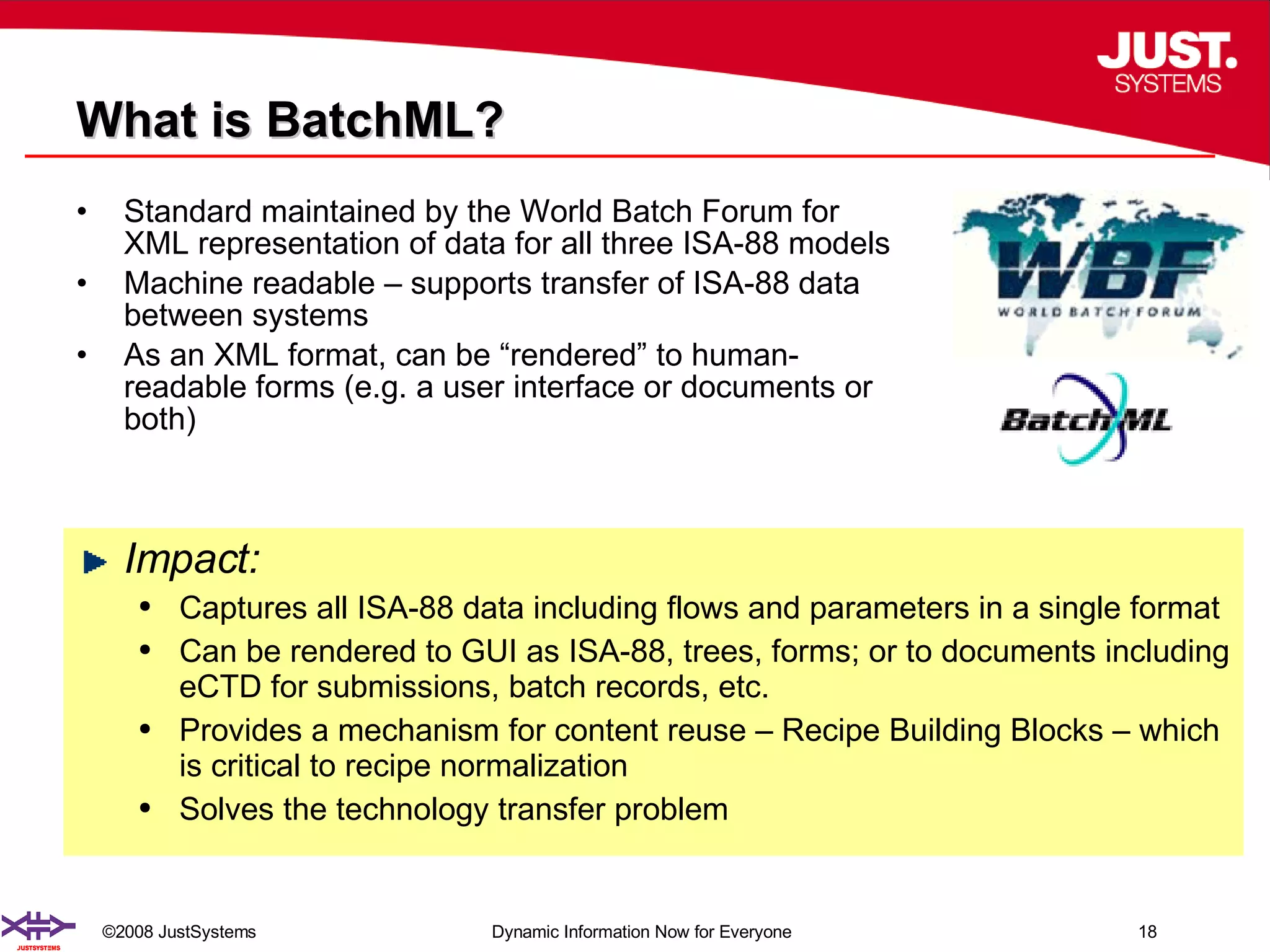
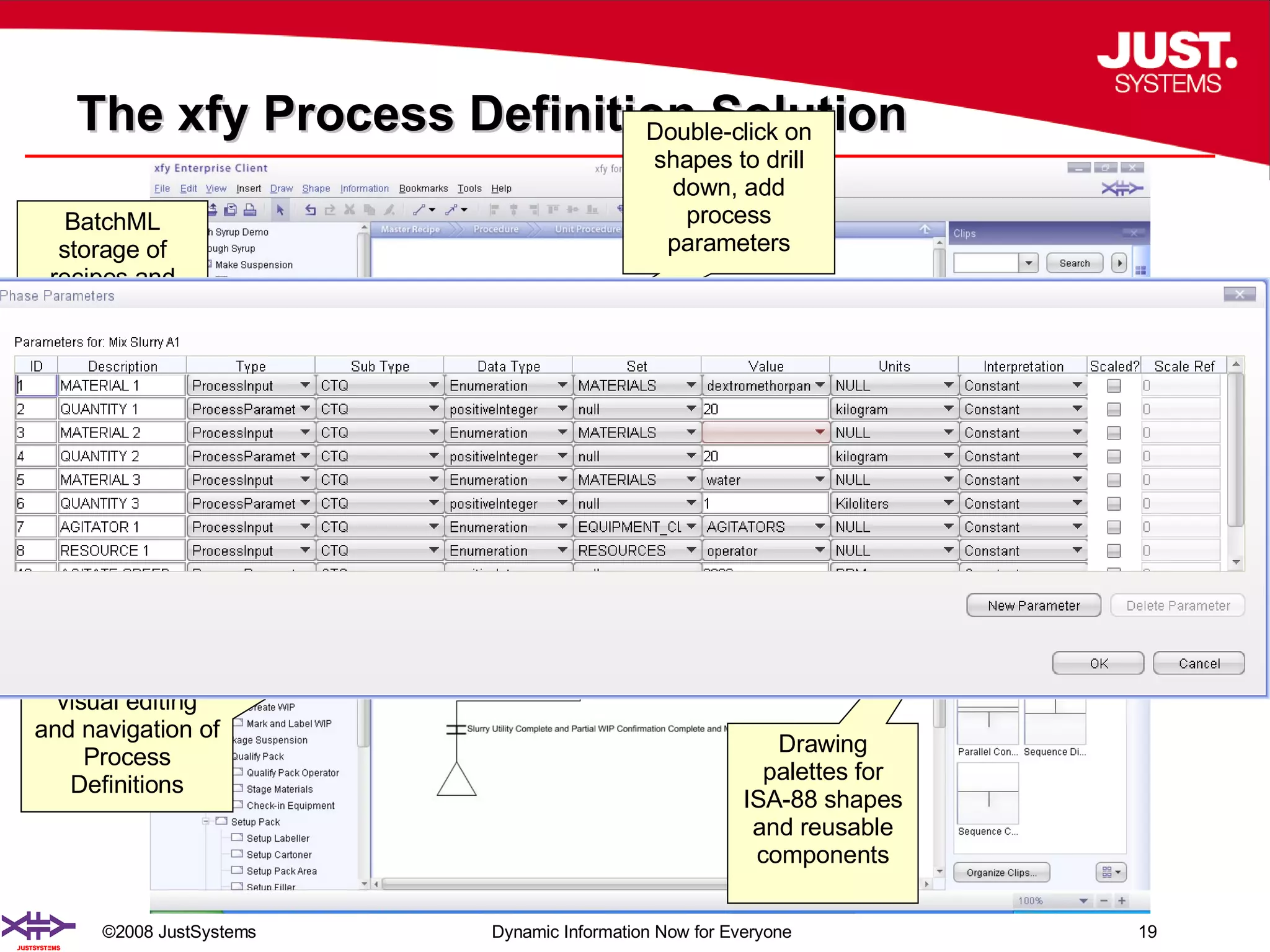
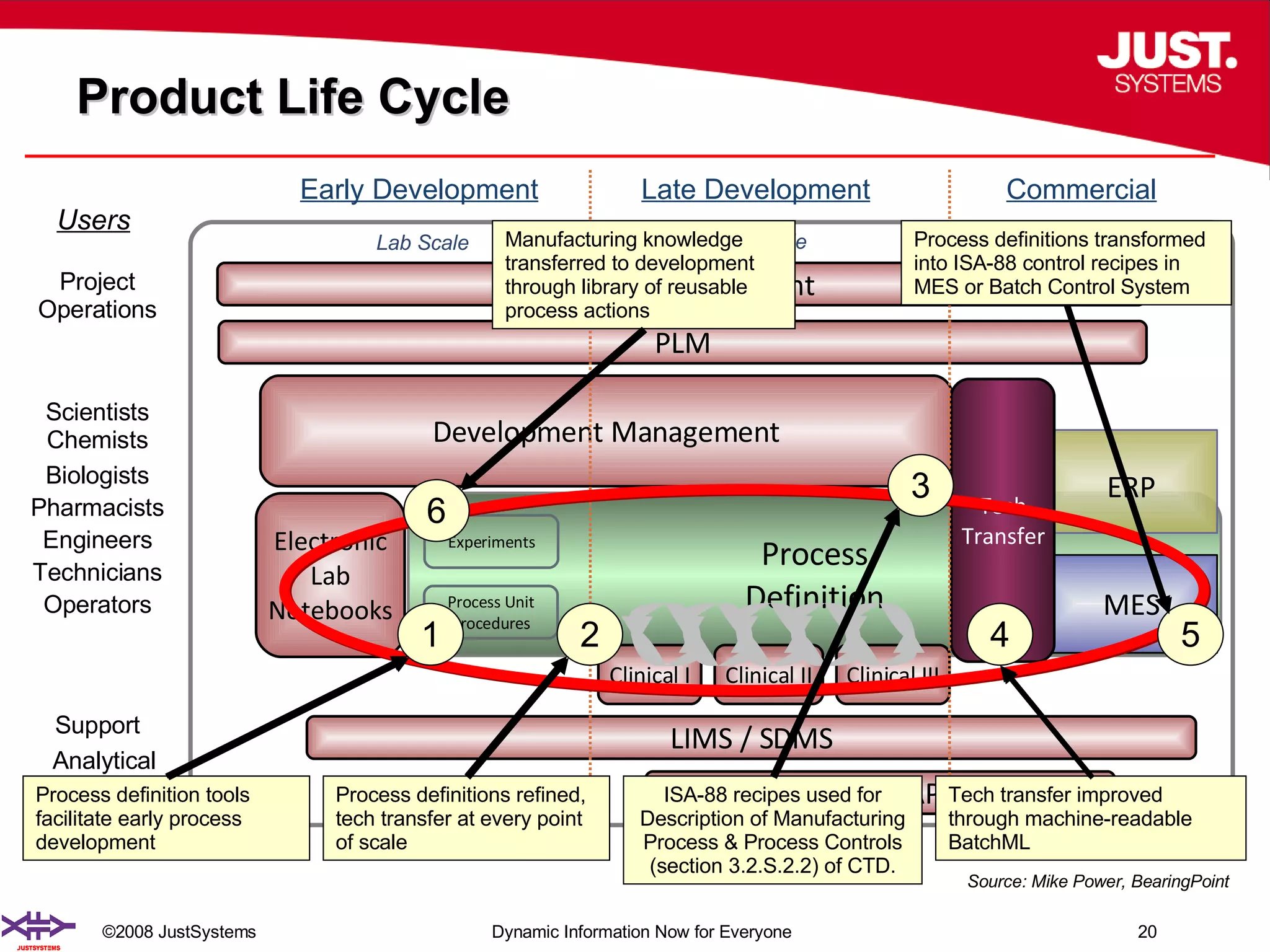
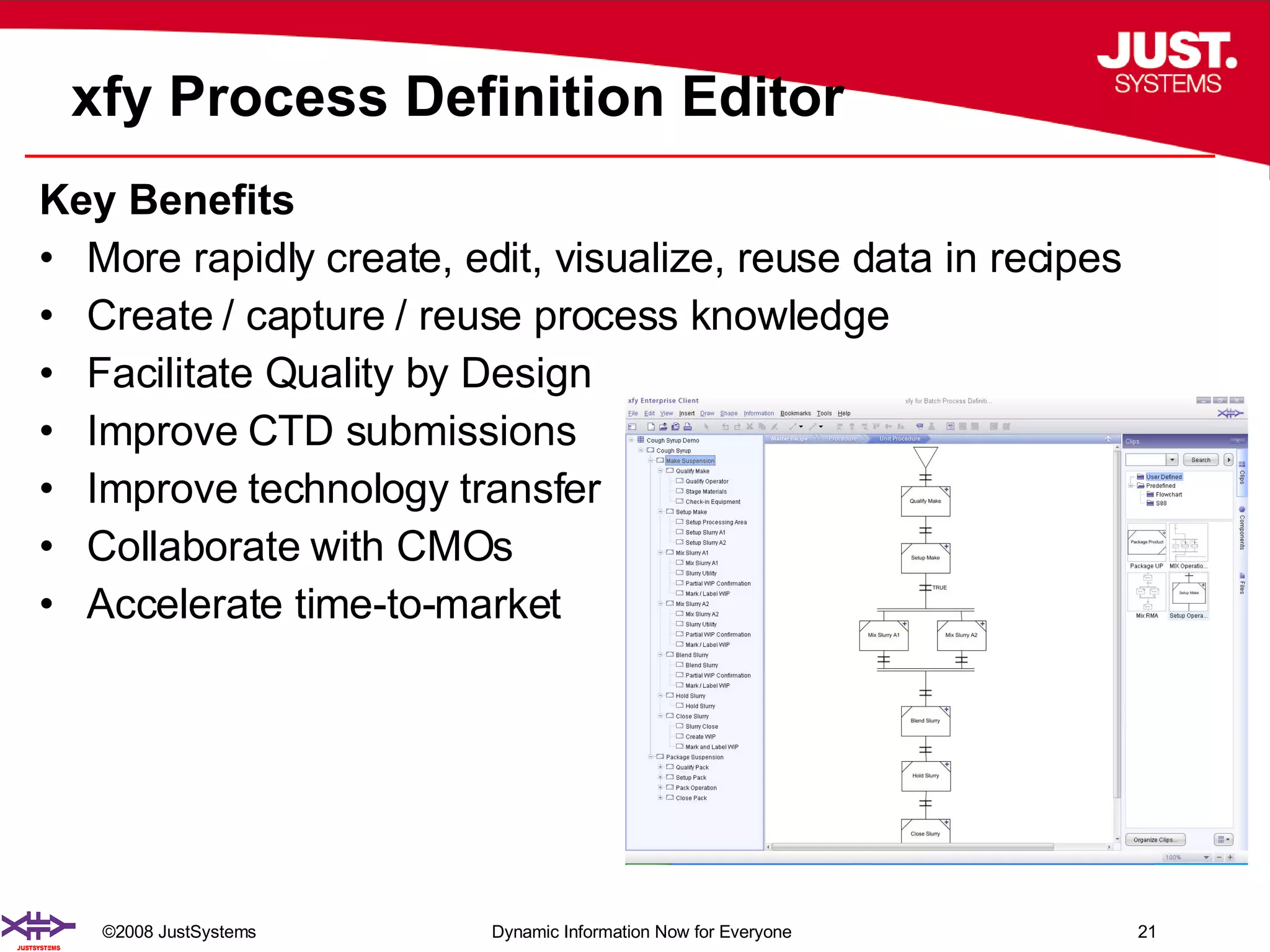
The document outlines the transformation of technology transfer and recipe management in life sciences from spreadsheets to standardized practices, emphasizing the need for improved agility in product development and reduced time-to-market. It proposes the use of common information models like ISA-88 to streamline process definitions, enhance knowledge management, and improve technology transfer across manufacturing stages. The xfy process definition solution is introduced as a means to facilitate these changes, promoting quality by design and accelerating product launch timelines.

![For more information Visit our website: http:// na.justsystems.com/lifesciences Email us: [email_address] Call us: (866) 793-1542](https://image.slidesharecdn.com/doctrain-ls-justsystems-wlodarczyk-tech-transfer-final-1213056326632281-8/75/Transforming-Technology-Transfer-and-Recipe-Management-From-Spreadsheets-to-Standardized-Practices-23-2048.jpg)