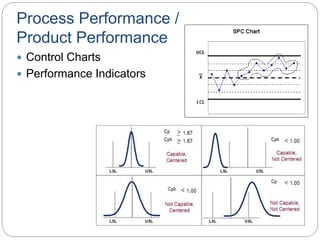
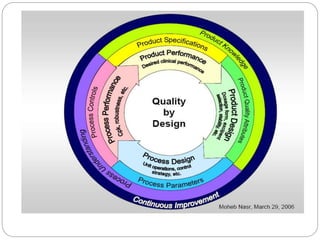
This document discusses key concepts of Quality by Design (QbD) including:
- QbD was first outlined by quality expert Joseph Juran and emphasizes removing failures to create customer satisfaction and designing in quality.
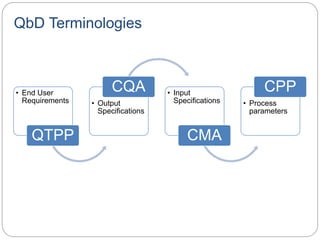
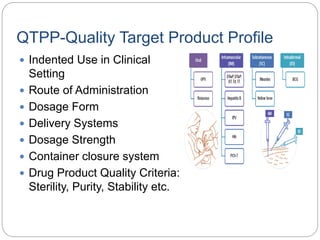
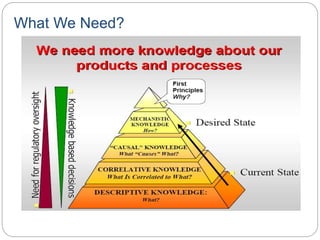
- The ICH definition of QbD is a systematic approach to development beginning with predefined objectives and emphasizing product and process understanding.
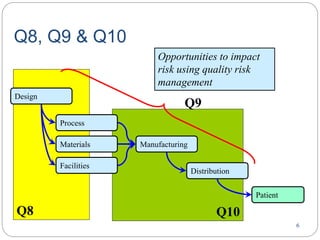
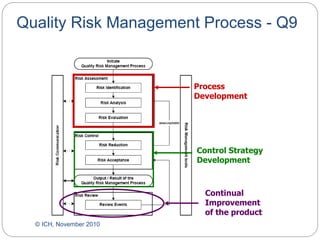
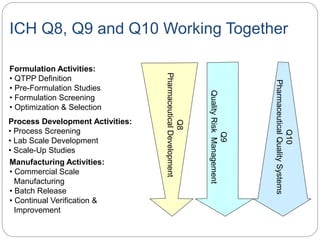
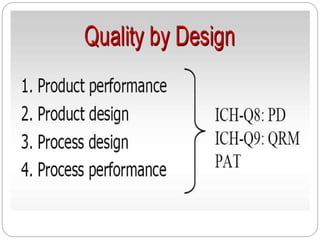
- ICH Q8, Q9, and Q10 provide guidance on applying science and risk-based approaches to pharmaceutical development and quality systems.
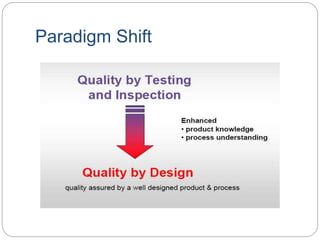
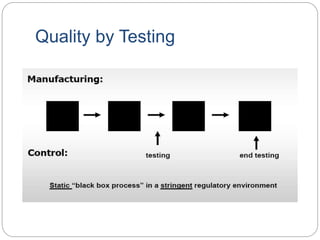
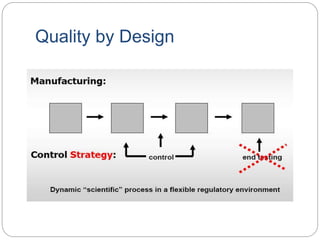
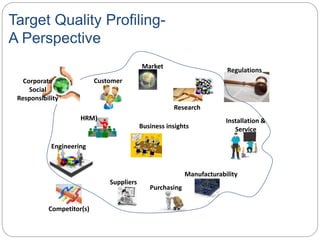
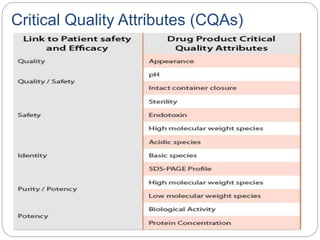
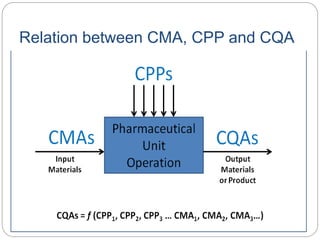
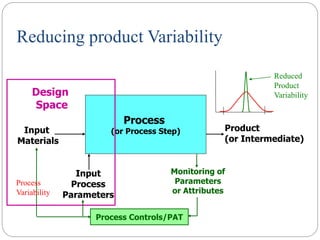
- QbD represents a paradigm shift from "quality by testing" to designing quality in based on an understanding of materials, processes, and how they impact critical quality attributes.