toaz.info-dll-physical-science-second-semester-pr_93130c7283e481d301c826362d3bc79b.pdf
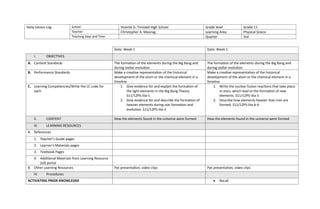
- 1. Daily Lesson Log School Vicente D. Trinidad High School Grade level Grade 11 Teacher Christopher A. Masirag Learning Area Physical Sciece Teaching Days and Time Quarter 3rd Date: Week 1 Date: Week 1 I. OBJECTIVES A. Content Standards The formation of the elements during the Big Bang and during stellar evolution The formation of the elements during the Big Bang and during stellar evolution B. Performance Standards Make a creative representation of the historical development of the atom or the chemical element in a timeline Make a creative representation of the historical development of the atom or the chemical element in a timeline C. Learning Competencies/Write the LC code for each 1. Give evidence for and explain the formation of the light elements in the Big Bang Theory. S11/12PS-IIIa-1 2. Give evidence for and describe the formation of heavier elements during star formation and evolution. S11/12PS-IIIa-2 1. Write the nuclear fusion reactions that take place in stars, which lead to the formation of new elements. S11/12PS-IIIa-3 2. Describe how elements heavier than iron are formed. S11/12PS-IIIa-b-4 II. CONTENT How the elements found in the universe were formed How the elements found in the universe were formed III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation, video clips Ppt presentation, video clips IV. Procedures ACTIVATING PRIOR KNOWLEDGE Recall
- 2. Guided Inquiry Strategy Film clip on the formation of the elements during the Big Bang and during stellar evolution Giving of guide questions for the film/video clip Video clip presentation ACQUIRING NEW KNOWLEGDE Discussion of the evidence for the formation of the light elements in the Big Bang theory Give evidence for and explain the formation of heavier elements during star formation and evolution Write the nuclear fusion reactions that takes place in stars, which lead to the formation of new elements Describe how elements heavier than iron formed ABSTRACTION Group work. Make a comic strip. Summing-it-up (Ask a volunteer to summarize the lesson) APPLICATION/EVALUATION Journal Writing Quiz Think-Pair-Share (Think of an application/s of the lesson learned today and share it with a partner) Quiz V. REMARKS The performance standard will be attained within the first three weeks. VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work?
- 3. F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: week 2 Date: week 2 I. OBJECTIVES A. Content Standards How the concept of the atom evolved from Ancient Greek to the present How the concept of the atom evolved from Ancient Greek to the present B. Performance Standards Make a creative representation of the historical development of the atom or the chemical element in a timeline Make a creative representation of the historical development of the atom or the chemical element in a timeline C. Learning Competencies/Write the LC code for each 1. Describe the ideas of the Ancient Greeks on the atom. S11/12PS-IIIa-b-5 2. Describe the ideas of the Ancient Greeks on the elements. S11/12PS-IIIa-b-6 3. Describe the contributions of the alchemists to the science of chemistry. S11/12PS-IIIb-7 1. Point out the main ideas in the discovery of the structure of the atom and its subatomic particles. S11/12PS-IIIb-8 2. Cite the contributions of J.J. Thompson, Ernest Rutherford, Henry Mosely, and Neils Bohr to the understanding of the structure of the atom. S11/12PS-IIIb-9 3. Describe the nuclear model of the atom and the location of its major components (protons, neutrons, and electrons). S11/12PS-IIIb-10 II. CONTENT How the idea of the atom, along with the idea of the elements evolved How the idea of the atom, along with the idea of the elements evolved III. LEARNING RESOURCES C. References
- 4. 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation Ppt presentation IV. Procedures ACTIVATING PRIOR KNOWLEDGE Sharing of the reading assignment Group Game/Quiz Bee ACQUIRING NEW KNOWLEGDE Large group discussion. (The teacher will facilitate the discussion in the class.)The discussion should tackle the following: A. ideas of the Ancient Greeks on the atom and elements B. contributions of the alchemist to the science of chemistry Worksheets Reporting Point out the ideas in the discovery of the structure of the atom and its subatomic particles. Cite the contributions of J.J. Thompson, Ernest Rutherford, Henry Moseley, and Neils Bohr to the understanding of the structure of the atom. Describe the nuclear model of the atom and the location of its major components (protons, neutrons, and electrons) ABSTRACTION What possible concepts of atom and elements may we have today if the Ancient Greeks did not thought of it? Make a list of main events that leads to the discovery of the structure of atom and its subatomic particles. APPLICATION/EVALUATION How can you share the knowledge learned in this lesson with your family and friends? Quiz. What is/are the importance of the concepts of atom and its subatomic particles in our world today? Explain. C. REMARKS D. REFLECTION A. No. of learners who earned 80% in the evaluation
- 5. B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: Week 3 Date: Week 3
- 6. I. OBJECTIVES A. Content Standards How the concept of the atom evolved from Ancient Greek to the present How the concept of the atom evolved from Ancient Greek to the present B. Performance Standards Make a creative representation of the historical development of the atom or the chemical element in a timeline Make a creative representation of the historical development of the atom or the chemical element in a timeline C. Learning Competencies/Write the LC code for each 1. Explain how the concept of atomic number led to the synthesis of new elements in the laboratory. S11/12PS-IIIb-11 2. Write the nuclear reactions involved in the synthesis of new elements. S11/12PS-IIIb-12 1. Cite the contribution of John Dalton toward the understanding of the concept of the chemical elements. S11/12PS-IIIc-13 2. Explain how Dalton’s theory contributed to the discovery of other elements. S11/12PS-IIIc-14 II. CONTENT How the idea of the atom, along with the idea of the elements evolved How the idea of the atom, along with the idea of the elements evolved III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation, video clips Ppt presentation, video clips IV. Procedures ACTIVATING PRIOR KNOWLEDGE Guided Inquiry Strategy - What is meant by atomic number? - How do scientist/chemist synthesize new elements in the laboratory? Ask the students to discuss the contribution of ancient scientists the development of the atomic theory. ACQUIRING NEW KNOWLEGDE Class Discussion. - The teacher will explain how concept of atomic number led to the synthesis of new elements in the laboratory. Group the class into 2 and discuss among themselves the assigned topic. After group sharing a discussion leader will present the concept they learned to the whole class.
- 7. - The teacher will give a board work on the students on writing the nuclear reactions involved in the synthesis of new elements. Group 1- Discuss the postulates and explain how these were able to explain the laws of chemical combination. Group 2- Discuss how the discovery of electrons affected Dalton’s atomic theory. ABSTRACTION There are new elements synthesized in the laboratory, due to nuclear development, higher elements have been discovered. What are the difference of these newly synthesized elements compared to the other elements in the representative groups? Dalton reasoned out that if atoms really exist, they must have a certain properties to augment the Laws of Chemical Combination. What are the properties of an atom according to John Dalton? APPLICATION/EVALUATION What are the importance of synthesizing new elements in the laboratory? Objective assessment. How would you differentiate Dalton’s model of the atom from Bohr’s model of the atom? V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve?
- 8. G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: Week 4 Date: Week 4 I. OBJECTIVES A. Content Standards How the uses of different materials are related to their properties and structures. How the uses of different materials are related to their properties and structures. B. Performance Standards C. Learning Competencies/Write the LC code for each 1. Determine if a molecule is polar or non-polar given its structure. S11/12PS-IIIc-15 2. Relate the polarity of a molecule to its structure. S11/12PS-IIIc-16 1. Describe the general types of intermolecular forces. S11/12PS-IIIc-d-17 2. Give the type of intermolecular forces in the properties of substance. S11/12PS-IIId-e-18 3. Explain the effect of intermolecular forces on the properties of substances. S11/12PS-IIId-e-19 II. CONTENT How the properties of mater relate to their chemical structure How the properties of mater relate to their chemical structure III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages Chang, R. Chemistry. 7th Edition. p.417-466 4. Additional Materials from Learning Resource (LR) portal
- 9. B. Other Learning Resources Ppt presentation Ppt presentation IV. Procedures ACTIVATING PRIOR KNOWLEDGE Present the basic assumptions of the VSEPR theory Show models of different molecular geometry A small drop of oil in water assumes a spherical shape. Explain. (this can be demonstrated by the teacher) {Hint: Oil is made up of nonpolar molecules, which tend to avoid contact with water.} ACQUIRING NEW KNOWLEGDE Have the class work on predicting molecular geometry of given compounds. Explain polar and nonpolar covalent bonding given its structure. Differentiate bond polarity from molecular polarity using H2O, CO2, CH4, NH2, SO2, and CHCl2 as examples. Group the class into 5 and assign each of the following type of intermolecular forces: 1. Dipole-dipole interaction 2. Dipole-induced dipole interaction 3. Ion-dipole interaction 4. Dispersion forces 5. Van der Waals forces Each group will discuss the given topic among themselves and prepare a ppt presentation for their output to share in the whole class. ABSTRACTION What are the general properties of polar compounds and of nonpolar compounds? What other factors are considered in determining molecular polarity? Explain the term “polarizability.” What kind of molecules tend to have high polarizabilities? What is the relationship between polarizability and intermolecular forces? APPLICATION/EVALUATION What is/are the application of the lesson you learned in your daily life? Discuss. Swimming coaches sometimes suggest that a drop of alcohol (ethanol) placed in an ear plugged with water “draws out the water.” Explain this action from a molecular point of view. V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson.
- 10. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: Week 5 Date: Week 5 I. OBJECTIVES A. Content Standards How the uses of different materials are related to their properties and structures. The relationship between the function and structure of biological macromolecules. B. Performance Standards C. Learning Competencies/Write the LC code for each 1. Explain how the uses of the following materials depend on their properties: a. medical implants, prosthesis; b. sports equipment; c. electronic devices; d. construction supplies for buildings and furniture; e. household gadgets. S11/12PS-IIId-e- 20 2. Explain how the properties of the above materials are determined by their structure. S11/12PS-IIId-e-21 Explain how the structures of biological macromolecules such as carbohydrates, lipids, nucleic acid, and proteins determine their properties and functions. S11/12PS-IIIe-22 II. CONTENT How the properties of mater relate to their chemical structure How the properties of mater relate to their chemical structure III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages
- 11. 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation, laboratory glass wares and chemicals Ppt presentation IV. Procedures ACTIVATING PRIOR KNOWLEDGE • The teacher will explain the fact that physical properties allow us to classify and identify chemical substances. • Physical properties are: colour, state of matter, melting and boiling point, density, solubility, electric and heat conductivity, volatility, surface tension, viscosity and capillary action. • The teacher demonstrates a few of the physical properties of different substances, e.g. compare the boiling points of water and pentane (or any non-polar organic substance); compare the viscosity of olive oil and water; compare the shiny surface of metals with the dull colours of ionic salts – the demonstration depends on the availability of chemicals in the school laboratory. The teacher ask the students to explain the difference between interatomic and intermolecular forces. Ask the students to classify covalent bonding as an interatomic- or intermolecular force. ACQUIRING NEW KNOWLEGDE Collaborative learning. (small group activity) Divide the class into five and assign the following materials (a. medical implants, prosthesis; b. sports equipment; c. electronic devices; d. construction supplies for buildings and furniture; e. household gadgets.) and let them explain how the properties of these materials affect their uses. Outputs will be presented in class. CLASSWORK ACTIVITY It is possible that only interatomic forces can be present in crystal lattice. A macro molecule is formed. a. Give two examples of macro molecules. b. In which phase will macro molecular structures most probably be at room temperature? (25 0 C)? c. Give a reason for your answer in question 4 b. d. Are the physical properties of matter related to interatomic - or intermolecular forces? After the presentation of classwork, the teacher further
- 12. discuss how the structures of biological macromolecules such as carbohydrates, lipids, nucleic acid, and proteins determine their properties and functions. ABSTRACTION The structures and properties of materials (crystals), such as melting point, density, and hardness are determined by the kinds of forces that hold the particles together. How can you classify materials based on the kind of forces that hold the particles together? How do the structures of the biological macromolecules unique to one another? Explain. APPLICATION/EVALUATION Investigate and explain the effects of intermolecular forces on evaporation, surface tension, solubility, boiling points and capillarity. How do the structures of the biological macromolecules determine their properties and functions? V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: 2 hours Week 6 Date: 7 hours Week 6-week 8 I. OBJECTIVES A. Content Standards The following aspects of chemical changes: The following aspects of chemical changes:
- 13. a. How fast a reaction takes place b. How much reactants are needed and how much products are formed in a reaction B. Performance Standards C. Learning Competencies/Write the LC code for each 1. Use simple collision theory to explain the effects of concentration, temperature, and particle size on the rate of reaction. S11/12PS-IIIf-23 2. Define catalyst and describe how it affects reaction rate. S11/12PS-IIIf-24 1. Calculate the amount of substances used or produced in a chemical reaction. S11/12PS-IIIf-h-25 • Perform stoichiometric calculations using balanced equations. • Convert any given units to required units. II. CONTENT How chemical changes takes place How chemical changes takes place III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation, video clips Ppt presentation, video clips IV. Procedures ACTIVATING PRIOR KNOWLEDGE Question and answer, Explanation 1. The collision theory explains why chemical reactions occur and why they take place at different rates. Give one term for each of the following descriptions. 1.1 A chemical substance that speeds the rate of a chemical reaction. 1.2 A collision in which the reacting particles have sufficient kinetic energy and the correct orientation. 1.3 The factor responsible for increasing the rate of reaction when a solid is broken into smaller pieces. 1.4 A measure of the average kinetic energy of the Simple Recall on Balancing of Chemical equation. The teacher should introduce the lesson by explaining what stoichiometric calculations are all about and the steps to be followed when dealing with stoichiometric calculations. (i) Stoichiometric calculations are calculations whereby number of moles, mass and volume of reactants and products of a chemical reaction are calculated. (ii) These calculations can be done by using a balanced chemical reaction.
- 14. particles in a gas. 2. The rate at which 50 mm piece of clean magnesium ribbon reacts with 20 cm3 hydrochloric acid (concentration of 1mol•dm-3 ) is determined by measuring the volume of hydrogen gas released during the reaction Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g) State how the rate of this reaction will be influenced if the experiment is repeated three times altering only ONE factor at a time as follows. (Simply state INCREASE, DECREASE, or REMAIN THE SAME) 2.1 The 50 mm piece of magnesium is filed to a powder. 2.2 20 cm3 of HCl of a 2mol•dm-3 concentration is used. 2.3 The mixture is cooled. 3. An iron nail will displace copper metal from a solution of copper sulphate. Give three ways in which the rate of this reaction can be increased SOLUTIONS 1. 1.1 catalyst 1.2 effective collision 1.3 surface area 1.4 temperature 2. 2.1 increase 2.2 decrease 2.3 decrease 3. a) Use iron powder instead of the nails. b) Heat the reaction mixture. c) Increase the concentration of the CuSO4 solution. (iii) Steps to be followed when doing stoichiometric calculations: Step 1: Make sure the equation is balanced. Step 2: Write down the molar ratio of the species present. Step 3: Calculate the number of moles using the given information. ACQUIRING NEW KNOWLEGDE Group work. Divide the class into 5 and assign each of the following topics. 1. The Collision Theory 2. State of division or surface area 3. The concentration of the reactants 4. The temperature of the reactants 5. The presence of a catalyst The teacher should give the learners the examples to be done in class. Examples: 1. Consider the following equation: Fe2O3 + H2 → Fe + H2O 1.1 Balance the equation. 1.2 How many moles of H2 are required to react with 50 g of Fe2O3? 1.3 Determine the mass of Fe produced from 50g of Fe2O3.
- 15. Let the students discuss the concept in their groups and assign a discussion leader to share it in class after. Alternative activity: Laboratory activity on the factors affecting chemical reaction 2. Consider the following equation: N2 + H2 → NH3 2.1 Balance the equation. 2.2 If 11,2 dm3 of hydrogen gas is placed in the reaction vessel at STP with excess nitrogen gas, determine the volume of ammonia that forms. The teacher will give other examples based on the three types of stoichiometric calculations based on the mole method. Alternative activity: Problem set will be given to the students. ABSTRACTION Ask learners about the main aspects of the lesson. On what law is stoichiometry based? Why is it essential to use balanced equations in solving stoichiometric problems? APPLICATION/EVALUATION How will you apply the learning you had today in your daily life? Problem solving. V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers?
- 16. Date: week 8 Date: week 9 I. OBJECTIVES A. Content Standards The following aspects of chemical changes: b. How much reactants are needed and how much products are formed in a reaction c. How much energy is involved in a reaction How energy is harnessed B. Performance Standards C. Learning Competencies/Write the LC code for each 1. Calculate percent yield of a reaction. S11/12PS- IIIh-26 2. Determine the limiting reactant in a reaction and calculate the amount of product formed. S11/12PSIII-h-27 3. Recognize that energy is released or absorbed during a chemical reaction. S11/12PSIII-i-28 1. Describe how energy is harnessed from different sources: a. Fossil fuel b. Biogas c. Geothermal d. Hydrothermal e. Batteries f. Solar cells g. Biomass S11/12PSIIIi-29 II. CONTENT How chemical changes takes place How chemical changes takes place III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation, video clips IV. Procedures
- 17. ACTIVATING PRIOR KNOWLEDGE Simple Recall. Let students write a balanced reaction and discuss the chemical reaction process. Film clips on harnessing energy in the environment. What source of energy do you think is the most efficient? ACQUIRING NEW KNOWLEGDE Group work Each group will be given problem set to solve. Each group will present their solution in class and discuss their solution. Collaborative learning Group the class into 7 and let each group describe how energy is harnessed from different sources. Assign each group the following: 1. Fossil fuel 2. Biogas 3. Geothermal 4. Hydrothermal 5. Batteries 6. Solar cells 7. Biogas Let each representative per group to share their outputs in class. ABSTRACTION Ask learners about the importance of knowing the concept of limiting reagents. In our world today which rely on electrical energy to initiate most of our activities form home, school, work place and even everywhere, give some ways on how we can maximize/conserve our present energy today? APPLICATION/EVALUATION How will you apply the learning you had today in your daily life? Ask the students to look for a partner to answer the problem set will be given. Journal writing. Let students write a journal on energy conservation. V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who
- 18. have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: week 9 Date: week 10 I. OBJECTIVES A. Content Standards The properties and the mode of action of the following consumer products: a. Cleaning materials b. cosmetics The properties and the mode of action of the following consumer products: a. Cleaning materials b. Cosmetics B. Performance Standards Make either a poster, a flyer, or a brochure on a product (such as fuels, household, or personal care products) indicating its uses, properties, mode of action, and precaution Make either a poster, a flyer, or a brochure on a product (such as fuels, household, or personal care products) indicating its uses, properties, mode of action, and precaution C. Learning Competencies/Write the LC code for each 1. Give common examples of cleaning materials for the house and of personal care. S11/12PSIIIi-j-30 2. From the products labels, identify the active ingredient(s) of cleaning products used at home. S11/12PSIIIi-j-31 1. Give the use of other ingredients in cleaning products. S11/12PSIIIi-j-32 2. Give common examples of personal care products used to enhance the appearance of the human body. S11/12PSIII-j-33 II. How chemistry contributes to the understanding of household and personal care products How chemistry contributes to the understanding of household and personal care products
- 19. III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal C. Other Learning Resources Ppt presentation, video clips IV. Procedures ACTIVATING PRIOR KNOWLEDGE Let students name common examples of cleaning materials for the house and for personal care. (The teacher can also give an advance assignment to let the students bring product labels of household materials and/or personal care in class.) Group work Divide the class into 5 and let them create an advertisement on any of the following cleaning agent, personal care products, and other household products. Let them present their outputs. ACQUIRING NEW KNOWLEGDE Collaborative learning Group the students into 8 (depending on the size of the class) and let them study the product labels they have. Let them study what are the main components of each of the labels they have. Each group will present their outputs in class. (The teacher may give this as an advance assignment) Partner-in-learning Ask the students to find a partner and make a library/internet search on the use of the different ingredients of any household products and how personal care products use to enhance the appearance of the human body. Let them present their output using powerpoint presentation. ABSTRACTION Ask learners about the main aspects of the lesson. Of the different advertisement in print ads, television, and internet, how are you being conveyed to purchase their product? APPLICATION/EVALUATION How will you apply the learning you had today in your Make either a poster, a flyer, or a brochure on a product
- 20. daily life? (such as fuels, household, or personal care products) indicating its uses, properties, mode of action, and precaution. V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers? Date: week 10 Date: week I. OBJECTIVES A. Content Standards The properties and the mode of action of the following consumer products: a. Cleaning materials b. cosmetics c. Performance Standards Make either a poster, a flyer, or a brochure on a product (such as fuels, household, or personal care products) indicating its uses, properties, mode of action, and precaution d. Learning Competencies/Write the LC code for 1. Identify the major ingredients of cosmetics such
- 21. each as body lotion, skin whitener, deodorants, shaving cream, and perfume. S11/12PSIIIj-34 3. Explain the precautionary measures indicated in various cleaning products and cosmetics. S11/12PSIII-j-35 II. CONTENT How chemistry contributes to the understanding of household and personal care products III. LEARNING RESOURCES A. References 1. Teacher’s Guide pages 2. Learner’s Materials pages 3. Textbook Pages 4. Additional Materials from Learning Resource (LR) portal B. Other Learning Resources Ppt presentation, video clips IV. Procedures ACTIVATING PRIOR KNOWLEDGE Film clip/let the student see an advertisement of any cosmetic products. ACQUIRING NEW KNOWLEGDE Group work. (Assign each group a cosmetic product to study ahead of time) Assign each of the following to each group: a. Body lotion (Jergens/Johnsons Baby or any other brand) b. Skin whitener (any brand) c. Deodorant (any brand) d. Shaving cream (any brand) e. Perfume (any brand) Let each group discuss among themselves the precautionary measures indicated in the given cosmetic
- 22. product/s. ABSTRACTION Personal hygiene is very important in personality development. How can your confidence build up by using cosmetic products? APPLICATION/EVALUATION Group work Make either a poster, a flyer, or a brochure on a product (such as fuels, household, or personal care products) indicating its uses, properties, mode of action, and precaution V. REMARKS VI. REFLECTION A. No. of learners who earned 80% in the evaluation B. No. of learners who require additional activities for remediation who scored below 80%. C. Did the remedial lesson work? No. of learners who have caught up with the lesson. D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did these work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or localized materials did I use/discover which I wish to share with other teachers?