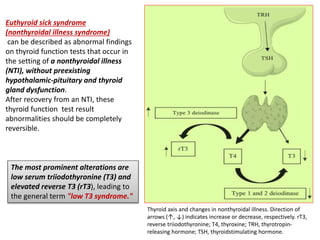
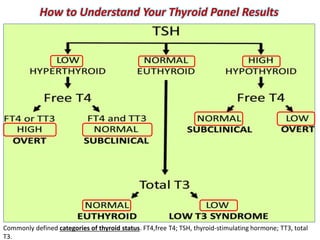
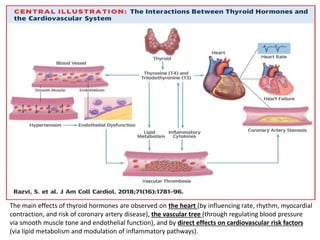
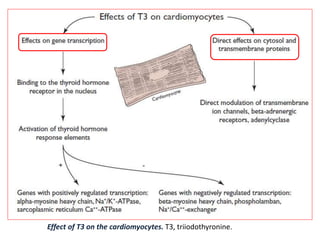
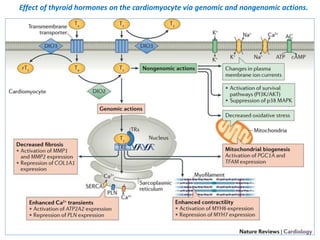
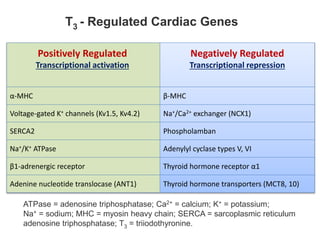
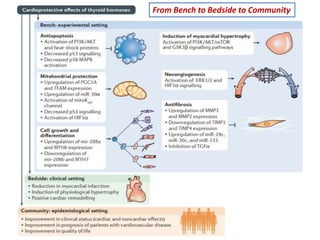
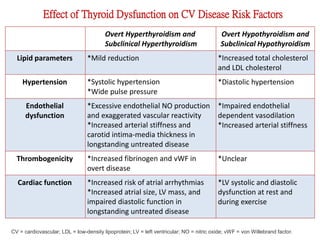
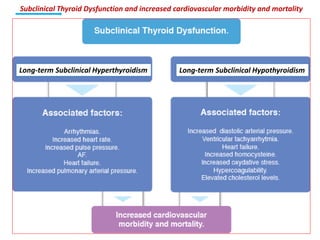
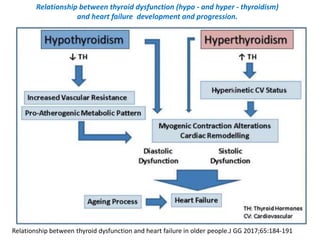
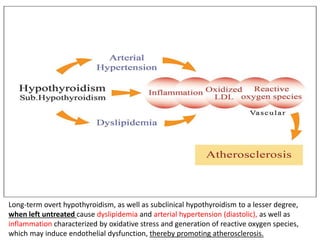
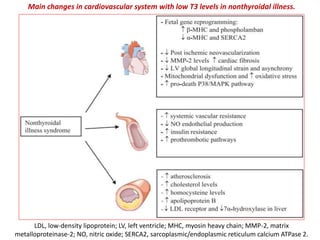
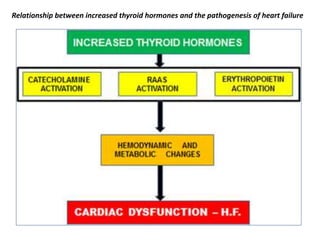
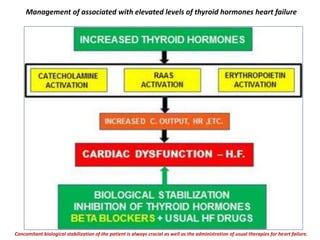
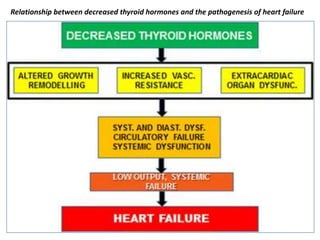
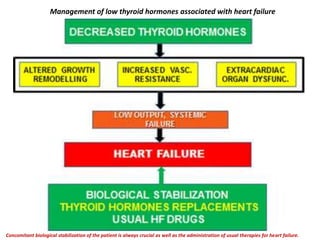
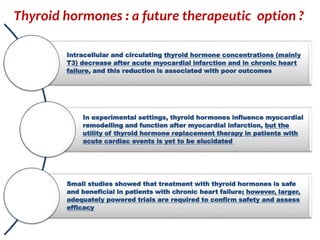
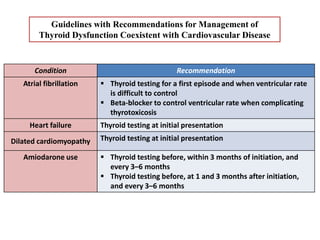
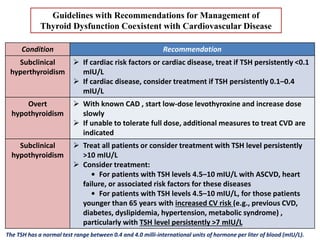
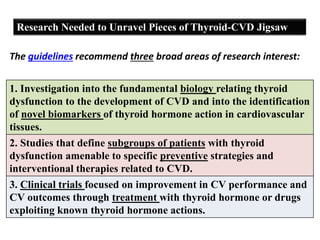
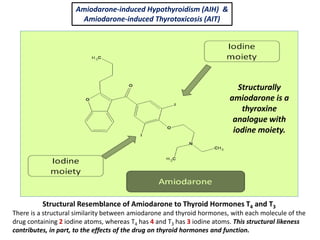
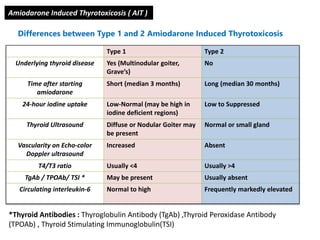
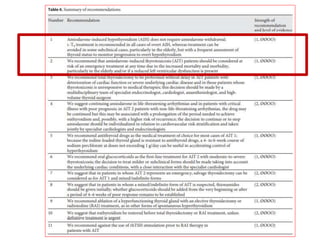
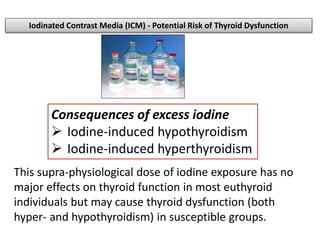
The document discusses the effects of thyroid hormones on cardiovascular function and diseases, detailing the relationship between thyroid dysfunction and cardiovascular risk factors, as well as potential therapeutic options. It highlights how thyroid hormones influence heart function, regulate blood pressure, and are linked to conditions like heart failure, emphasizing the need for guidelines in managing thyroid conditions alongside cardiovascular diseases. Additionally, it points to the necessity for future research to explore the biological connection between thyroid dysfunction and cardiovascular disease development.