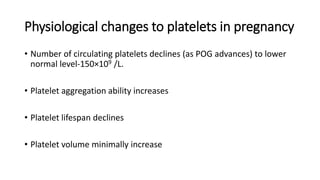
Thrombocytopenia with seizures in pregnancy can indicate serious conditions like preeclampsia/eclampsia, HELLP syndrome, TTP, or HUS. Preeclampsia is the most common cause, resulting from endothelial cell damage leading to platelet activation and coagulation. Management involves blood pressure control, seizure prevention with magnesium sulfate, and timely delivery. Differentiating these microangiopathies can be difficult but is important for targeting appropriate treatment like plasma exchange for TTP. A multidisciplinary approach and delivery optimization are crucial for improving maternal and neonatal outcomes.