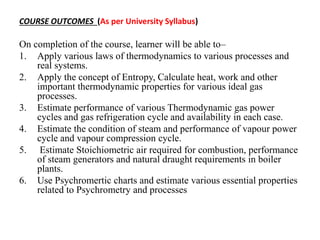
The document outlines the course objectives and outcomes for a Thermodynamics course. It includes 6 units: 1) Laws of Thermodynamics, 2) Entropy, 3) Thermodynamic Cycles, 4) Properties of Pure Substances, 5) Steam Generators, and 6) Psychrometry. The objectives are to understand key thermodynamic concepts like the laws of thermodynamics, entropy, enthalpy, cycles, and their real-world applications. The outcomes are that students will be able to apply these concepts to problems involving gases, steam, combustion, psychrometry and more.
![Matoshri College of Engineering & Research Centre,
Eklahare, Nashik
Program: Mechanical Engineering
Course: Thermodynamics[202043]
Class : Second Year Engineering
By
Mr. R.S.Patil](https://image.slidesharecdn.com/thermo-180801061310/75/Thermodynamics-Course-Objective-and-Course-Outcomes-1-2048.jpg)

![Course : Thermodynamics[202043]
Unit 1: Laws of thermodynamics
CO.1 Understand the role of the internal energy,
enthalpy, entropy, temperature, pressure and specific
volume thermodynamic properties
a) State and illustrate laws of Thermodynamics..
b) State and illustrate Zeroth law of thermodynamics
c) Student should able to understand energy equation and
its application
d) Student should to access thermodynamic property
thermodynamics table](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-3-320.jpg)
![CO.2 Explain the concepts of entropy,enthalpy and
Ideal gas equation and various gas Laws:
a) Student should able to understand Entropy and its
application
b) Student should able to understand Gas Laws
c) Student should able to understand thermodynamic
processes
Course : Thermodynamics[202043]
Unit2: Entropy](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-4-320.jpg)
![CO.3 Understand the role of thermodynamic cycles,
availability and irreversibility.
a) Student should able to quantify the behavior Gas
Power Cycles cycle
b) Student should able to understand concept of
Availability
c) Student should able to understand concept of
irreversibility and second law efficiency.
Course : Thermodynamics[202043]
Unit 3: Thermodynamic cycles](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-5-320.jpg)
![CO.4 Recognize and understand the different forms
of pure substances
a) Student should able to quantify the behavior
substances.
b) Student should able to understand working and use
of calorimeters.
c) Student should able to quantify the behavior
Thermodynamic Vapour Cycle
d) Student should able to read thermodynamics charts
and draw P-V and T-S diagrams
Course : Thermodynamics[202043]
Unit 4: Properties of Pure substances](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-6-320.jpg)
![CO.5 Student should able to quantify the
performance of Boiler
a) Student should able to understand combustion
phenomenon.
b) Be able to quantify the behavior of Boiler based on
the thermodynamic cycle, including the effect of
parameters.
Course : Thermodynamics[202043]
Unit 5: Steam Generators](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-7-320.jpg)
![CO.6 Be able to use Psychrometry processes in real
world engineering examples.
a) Student should able to understand basics of
Psychrometry.
b) Student should able to understand, Thermodynamics
of Human Body and Comfort Conditions .
Course : Thermodynamics[202043]
Unit 6: Psychrometry](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-8-320.jpg)
![Course : Thermodynamics[202043]
Course Objectives (As per University Syllabus)
1. Identify and use units and notations in Thermodynamics.
2. State and illustrate first and second laws of
Thermodynamics.
3. Explain the concepts of entropy, enthalpy, reversibility
and irreversibility.
4. Apply the first and second laws of Thermodynamics to
various gas processes and cycles.
5. To get conversant with properties of steam, dryness
fraction measurement, vapor processes and
Thermodynamic vapor cycles, performance estimation.
6. To get conversant with Psychrometric Charts,
Psychrometric processes, human comfort conditions.](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-9-320.jpg)

![CoM.1 Ability to apply various thermodynamics laws to
real system.
a) Student will be able to apply first and second law of
thermodynamics.
b) Student will be able to apply energy equations.
c) Student will be able to determine the reversibility or
irreversibility of a thermodynamic process.
Course : Thermodynamics[202043]
Unit1: Laws of thermodynamics](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-11-320.jpg)
![CoM.2 Understanding of the entropy of system and
ideal gas equations
a) An ability to calculate entropy changes in a processes
b) An understanding of the interrelationship between
ideal gas equations.
c) An understanding of the interrelationship between
thermodynamic processes.
Course : Thermodynamics[202043]
Unit 2: Entropy](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-12-320.jpg)
![CoM.3 An understanding of the interrelationship
between thermodynamic cycles.
a) Student should understand basics of Gas Power
Cycles.
b) Student should understand importance Gas
Refrigeration Cycle.
c) Student should understand importance Availability.
Course : Thermodynamics[202043]
Unit 3: Thermodynamic cycles](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-13-320.jpg)
![CoM.4 Ability to use Properties of Pure substances in
real thermodynamics problems.
a) Student should understand basics of steam.
b) Student should solve problems related to
thermodynamics diagrams.
c) Student should analyze Thermodynamic Vapour
Course : Thermodynamics[202043]
Unit 4: Properties of Pure substances](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-14-320.jpg)
![CoM.5 An ability to relate the characteristics of Steam
Generators.
a) Student should understand basics combustion
b) Student should solve problems related to boiler
performance
Course : Thermodynamics[202043]
Unit 5: Steam Generators](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-15-320.jpg)
![CoM.6 An ability to relate the characteristics of
Psychrometry.
a) Student should understandNumerical treatment using
Psychrometric chart
b) Student should understand, Thermodynamics of
Human Body and Comfort Conditions
Course : Thermodynamics[202043]
Unit 6: Psychrometry](https://image.slidesharecdn.com/thermo-180801061310/85/Thermodynamics-Course-Objective-and-Course-Outcomes-16-320.jpg)