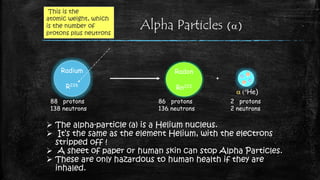
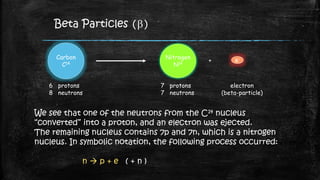
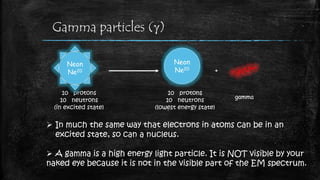
Thermionic emission is the process where heated electrons gain enough thermal energy to overcome the work function of a material, allowing them to flow from its surface. This occurs because the thermal energy given to charge carriers, such as electrons, overcomes the binding potential, or work function, of the material.