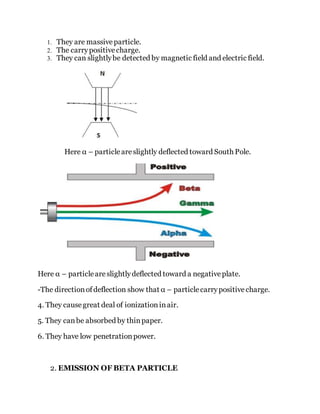
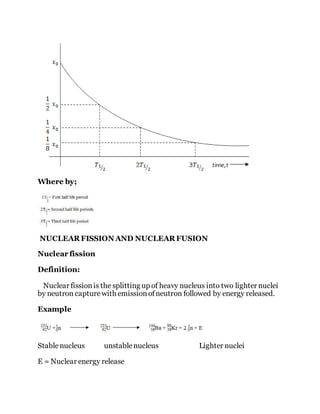
The document details the evolution of atomic theory, from Democritus' conception of indivisible atoms to modern understanding involving subatomic particles, including protons, neutrons, and quarks. It explains radioactivity, types of radioactive decay, and methods of detecting radiation, as well as the concept of half-life in radioactive materials. The document also covers artificial radioactive decay, highlighting methods of inducing it and calculations related to the half-life of various isotopes.