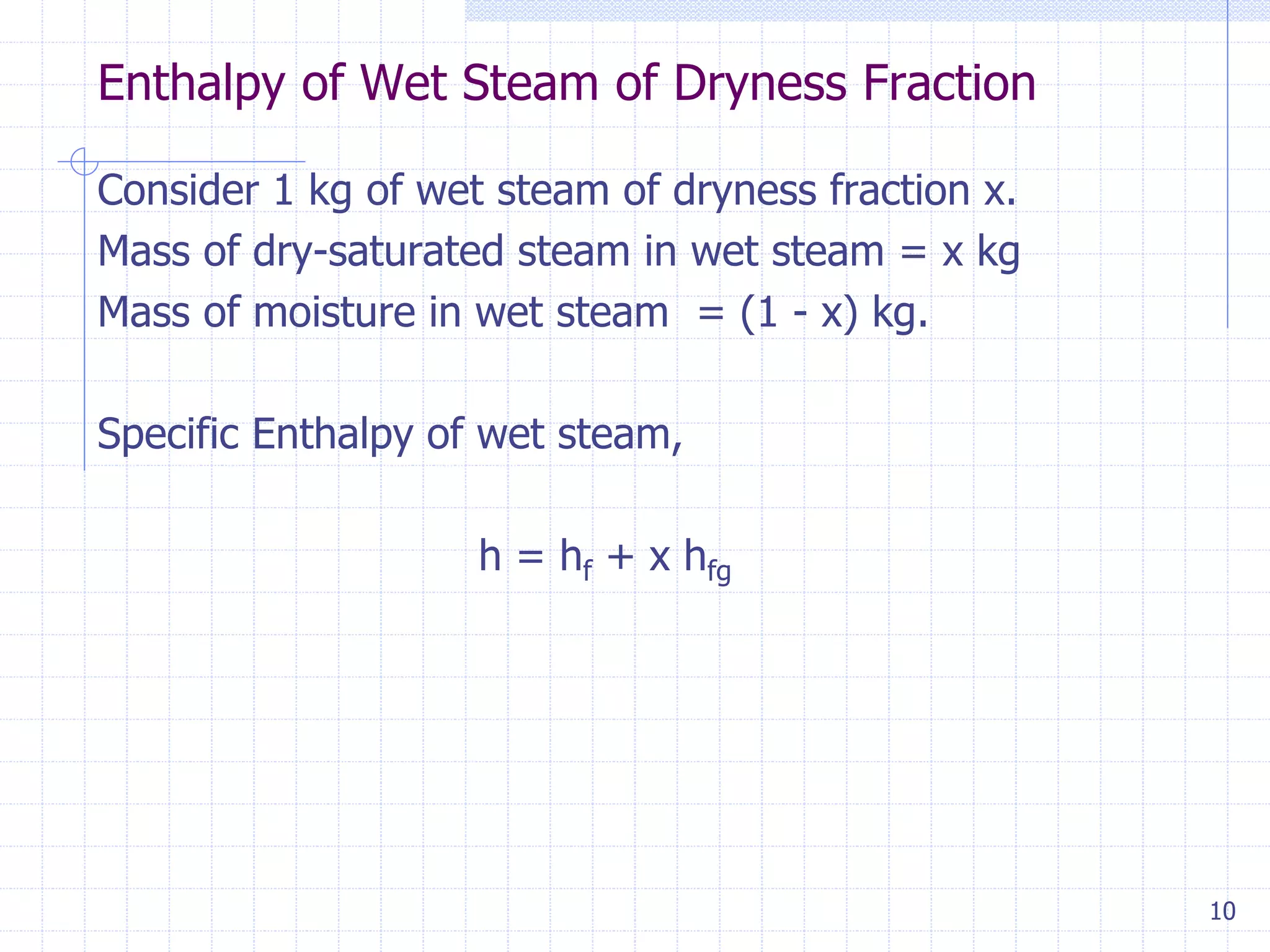
This document discusses properties of pure substances, specifically steam. It defines steam as water in its gaseous state or as a liquid-gas mixture. Steam is commonly used in steam engines and turbines for power generation. The document outlines key steam properties including specific sensible heat, latent heat of vaporization, enthalpy of dry-saturated steam, enthalpy of superheated steam, wet steam properties, dryness fraction, and enthalpy of wet steam as a function of dryness fraction. These properties are important thermodynamic concepts used to characterize the state and behavior of steam.