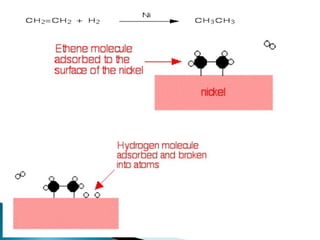
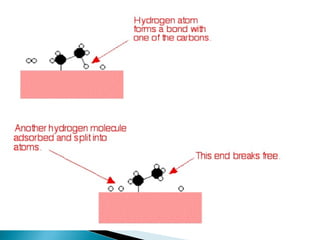
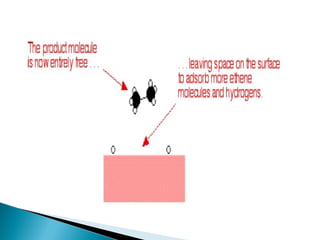
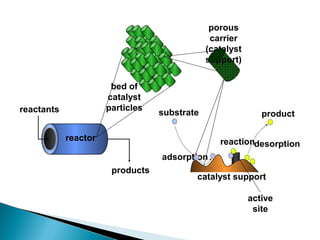
This document discusses the field of catalysis. It begins by noting that 24% of GDP comes from products that use catalysts in areas like food, fuels, chemicals and more. It then discusses the importance of catalysis in petroleum refining, chemical processes, and pollution control technologies. The document outlines the steps in a catalytic reaction and describes techniques for characterizing catalysts and testing their activity. It provides examples of applications of catalysis in industries like chemicals and environment. Recent trends discussed include catalysts for biodiesel and carbon nanotubes. The document concludes by anticipating future directions in catalysis related to alternative feedstocks, process control, and improvements in fuel cells.