Embed presentation
Download as PDF, PPTX

















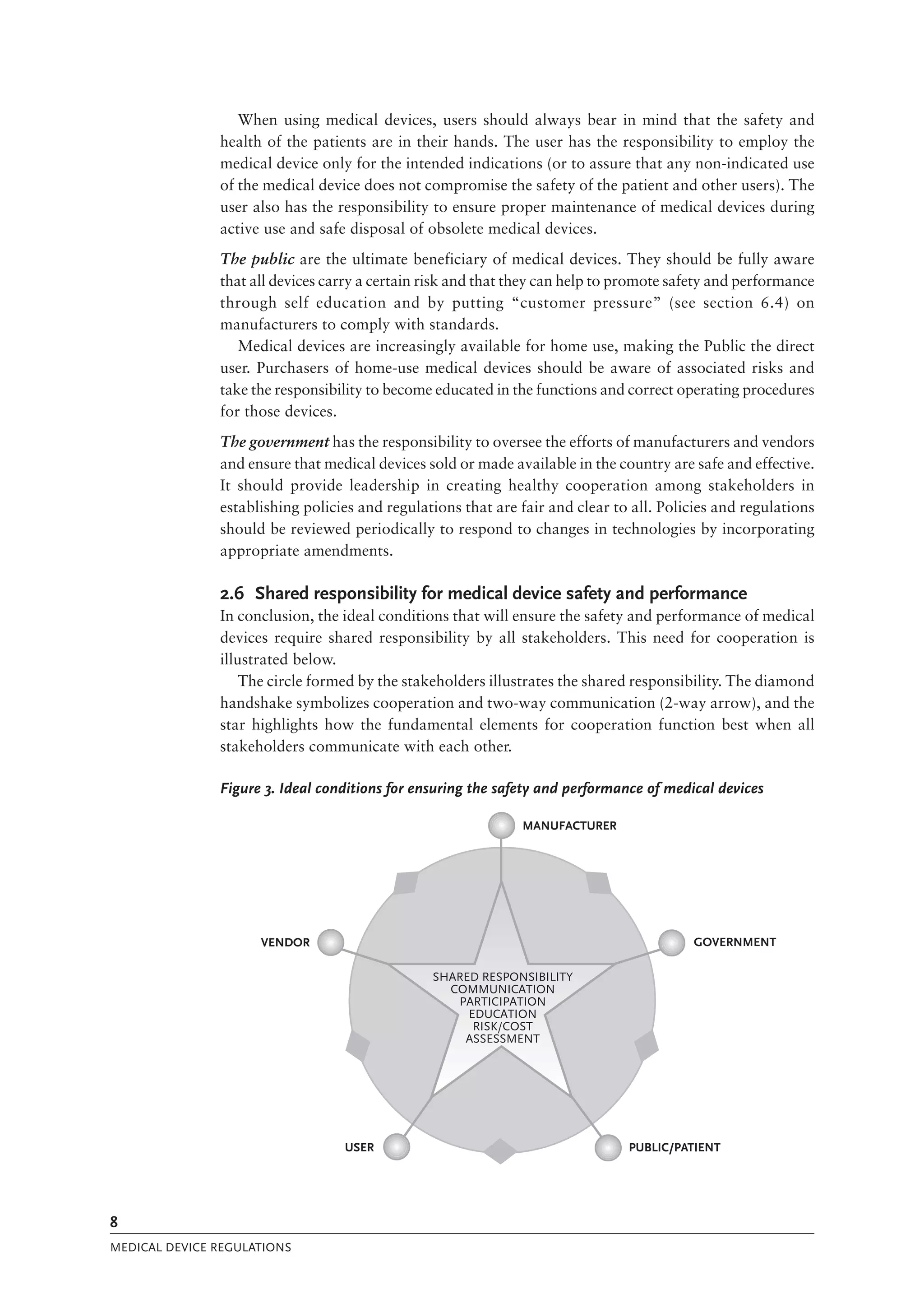

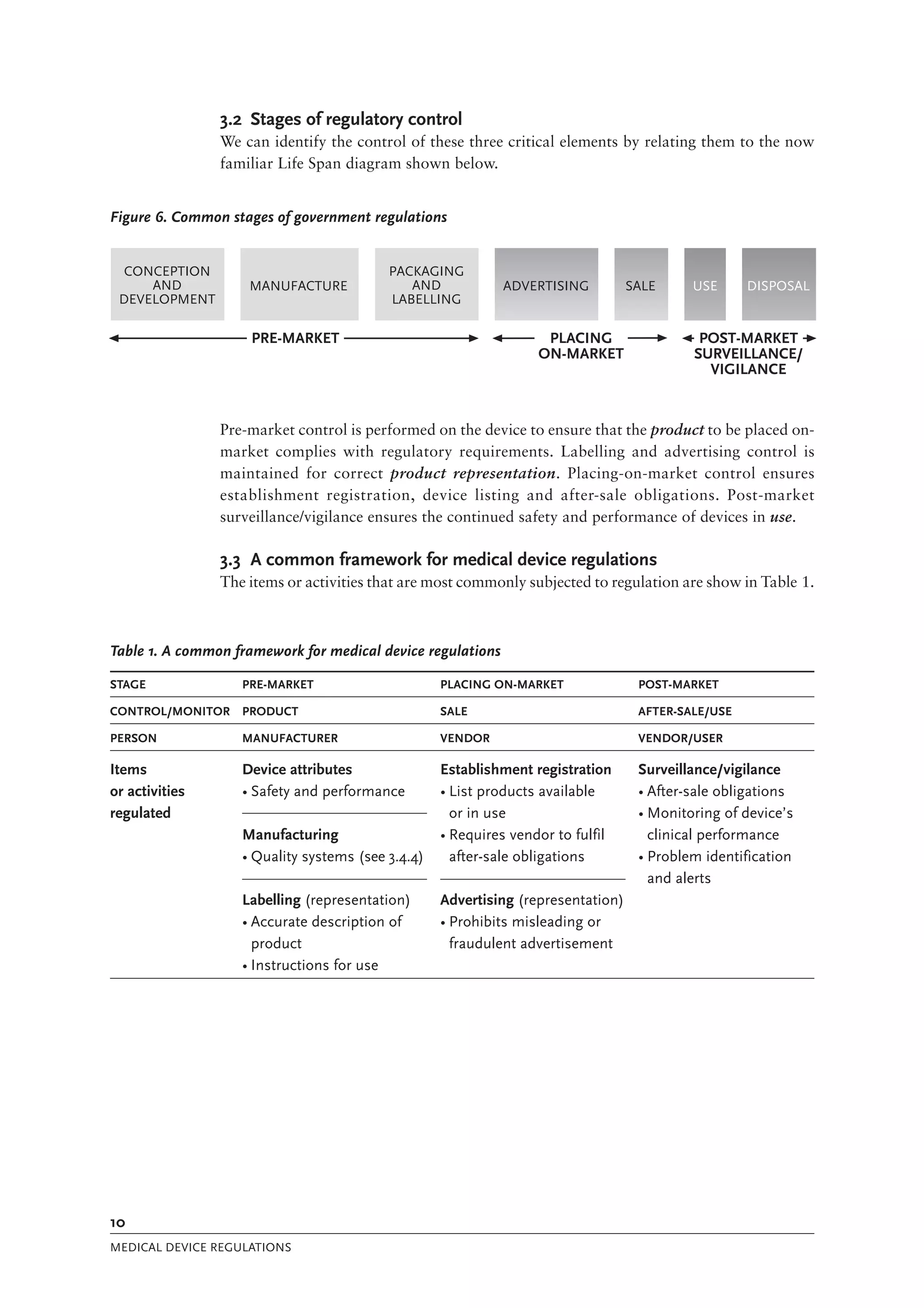
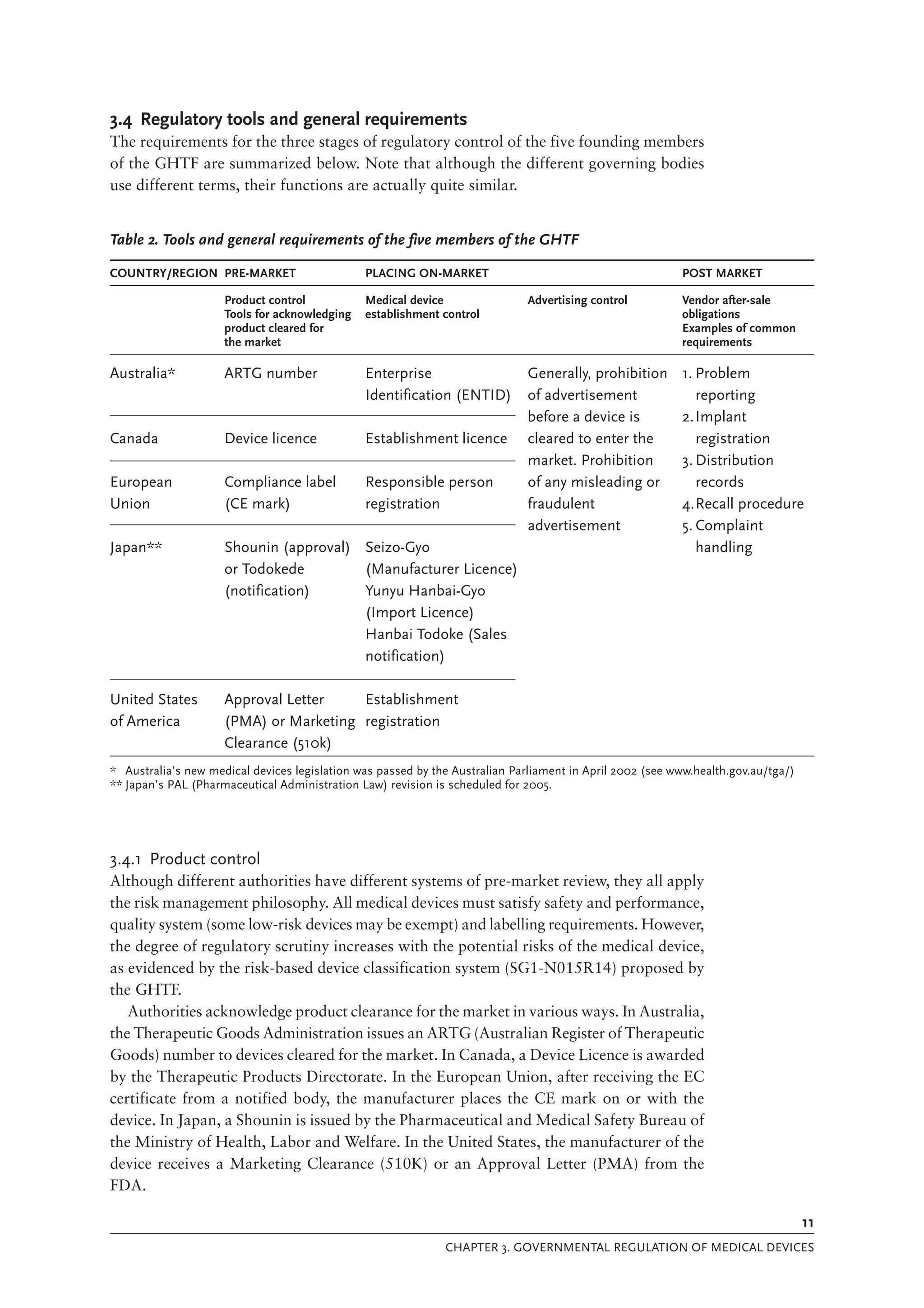


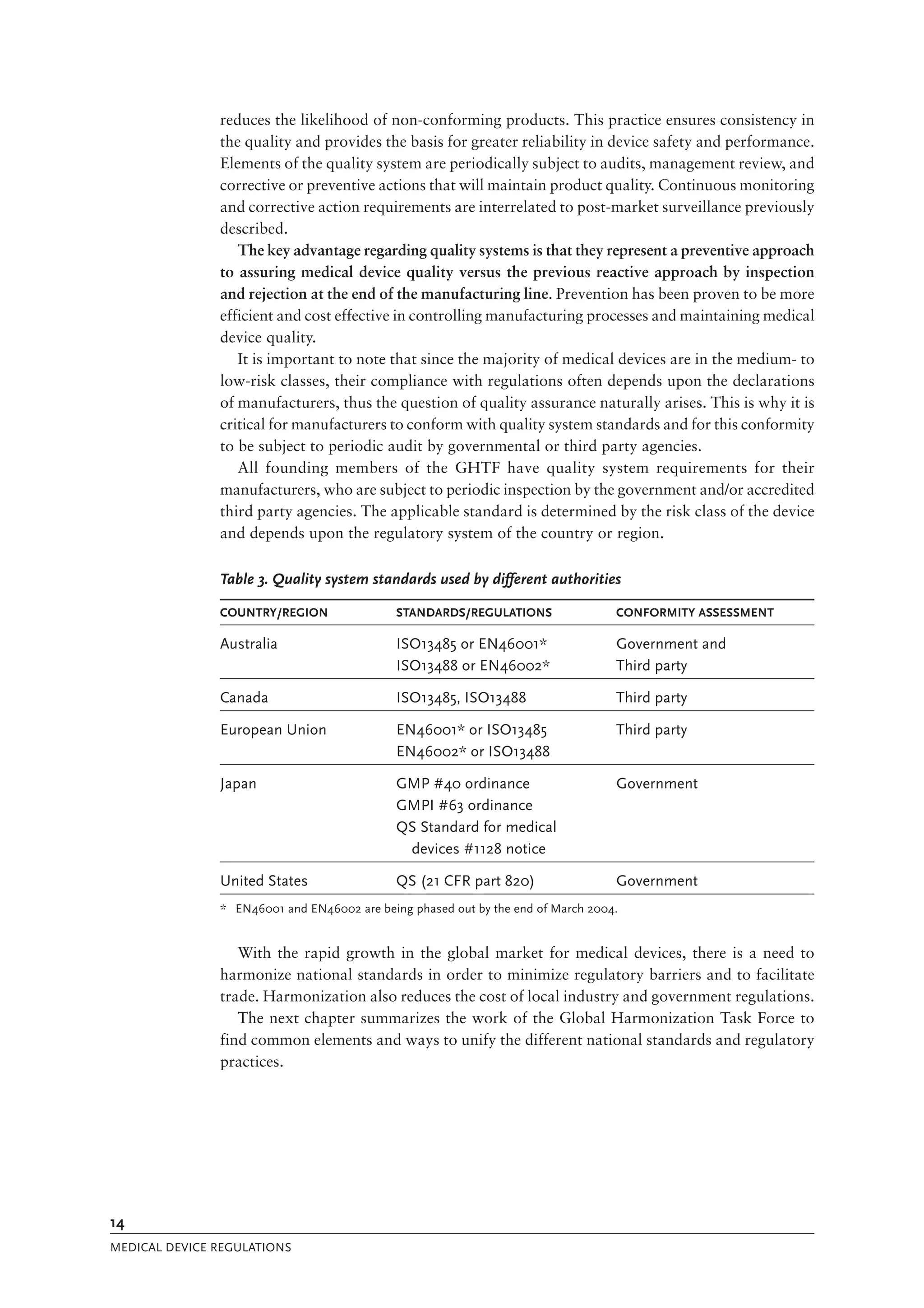












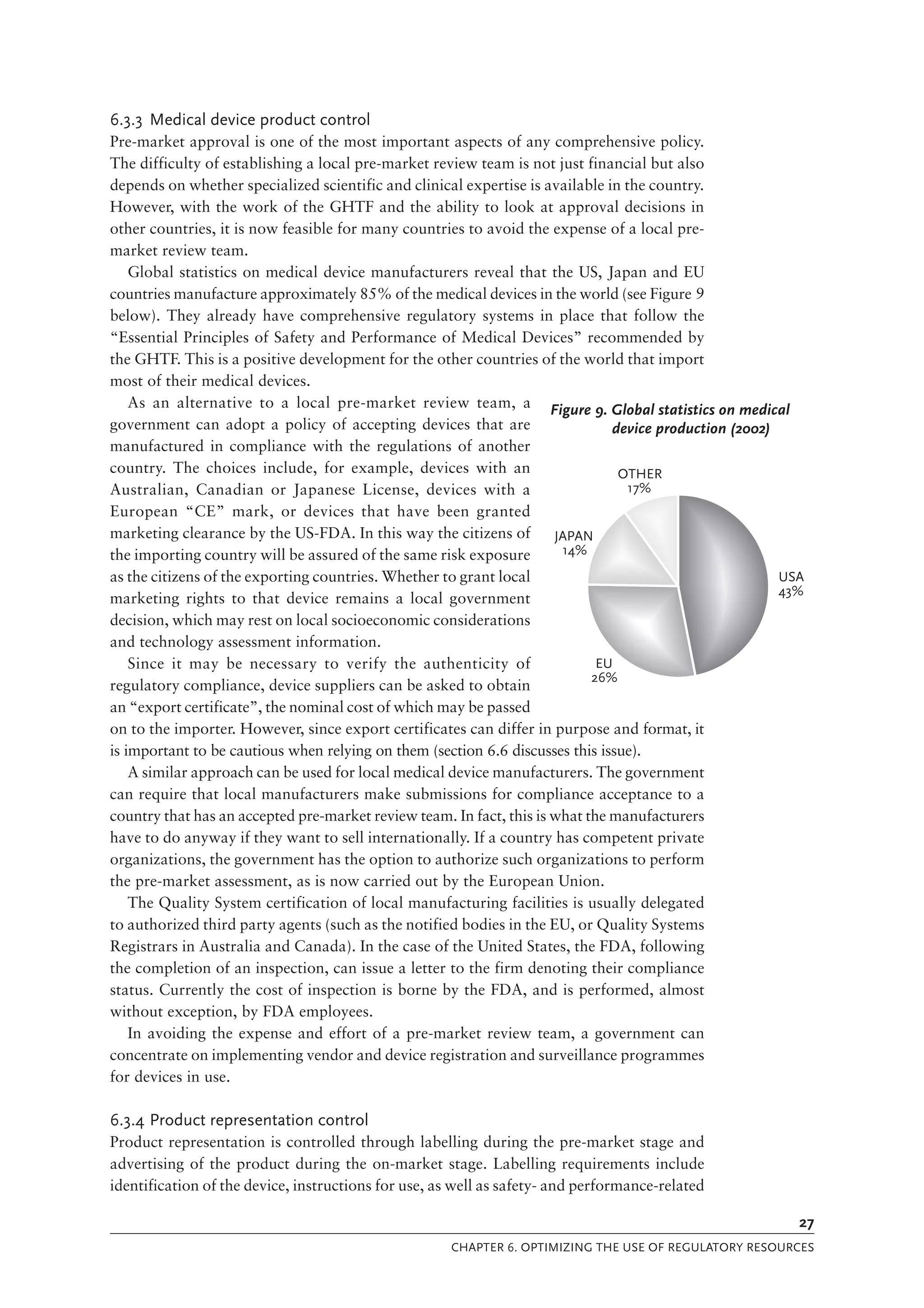












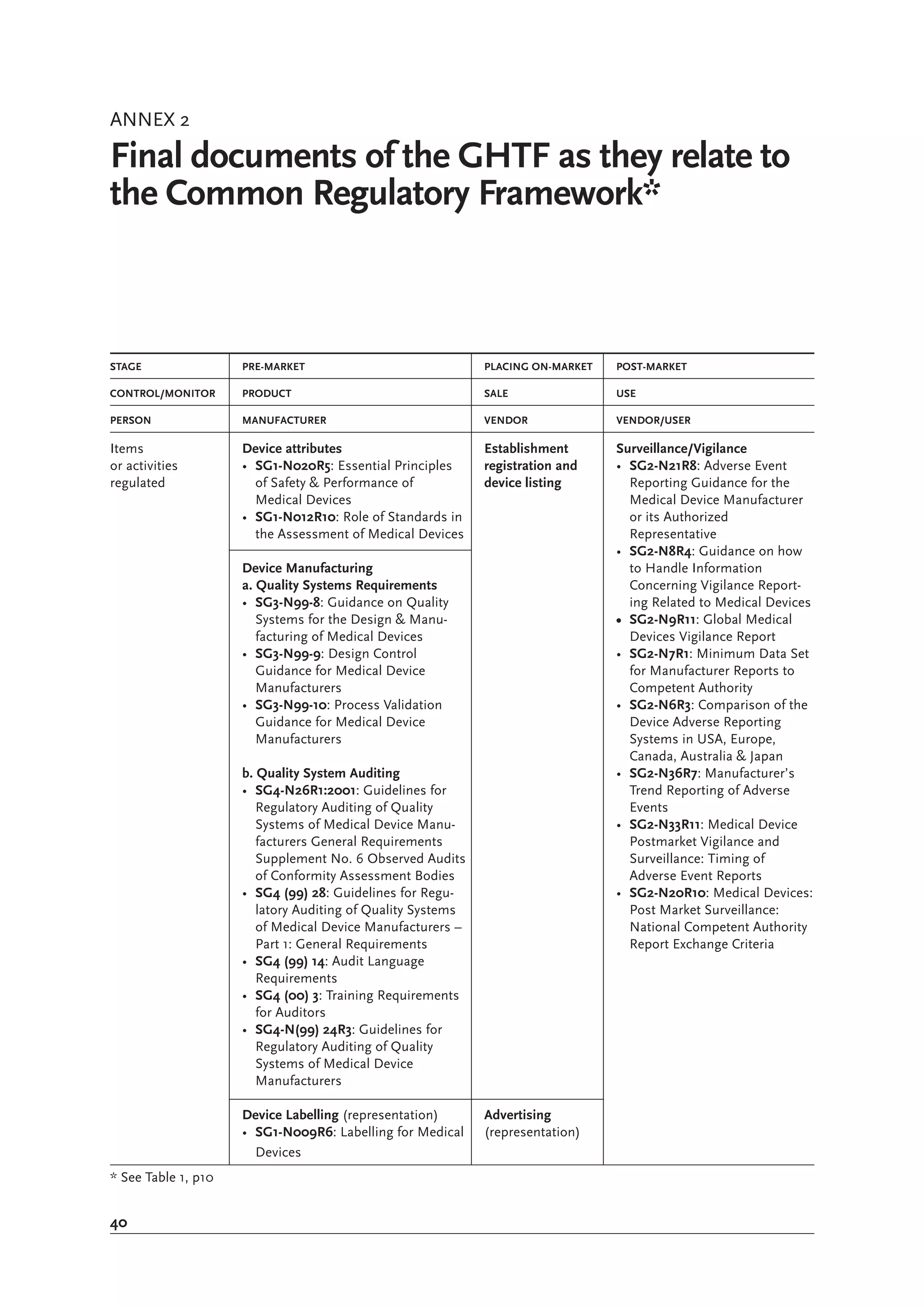


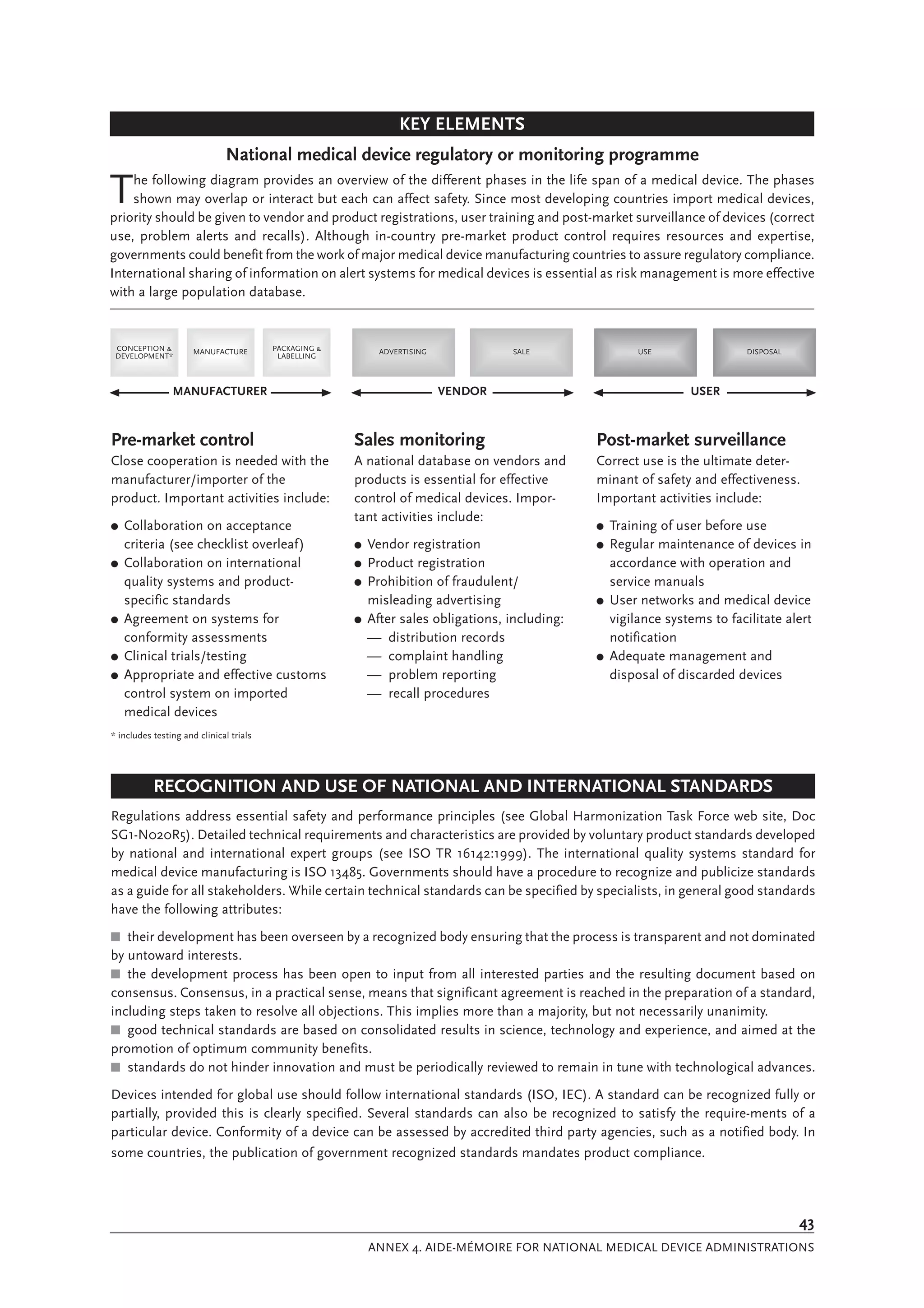

The document is a comprehensive guide on medical device regulations, providing an overview of global standards and principles established by the World Health Organization. It emphasizes the importance of safety, risk management, and harmonization of regulatory frameworks to ensure access to high-quality medical devices, especially in developing countries. The guide serves as a resource for member states aiming to enhance their regulatory systems for medical devices, outlining key components, responsibilities, and recommendations for effective regulation.





















































