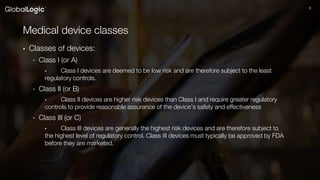
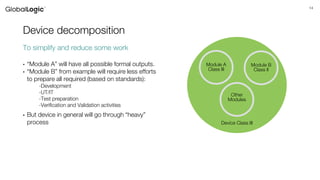
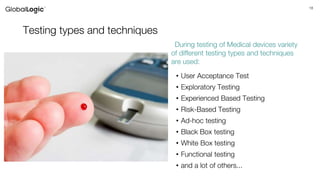
The document discusses the classification, regulation, and quality standards of medical devices as managed by the FDA. It outlines the definitions, risks associated with different device classes, and the necessary standards for effective medical device management and testing. Additionally, it emphasizes the importance of feasibility studies, documentation, and various testing techniques used in ensuring device safety and effectiveness.