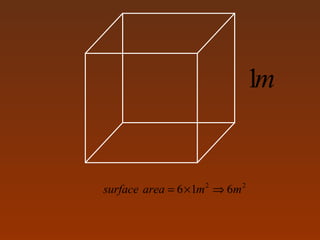
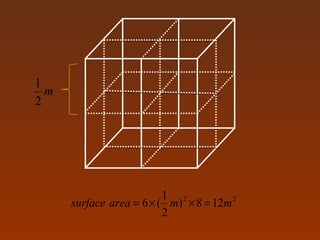
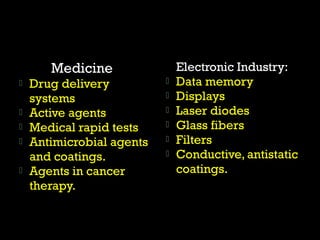
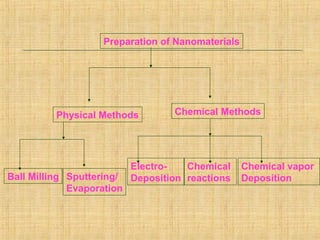
Matter and energy exist in various forms and interact in many ways. Nano materials are materials that have at least one dimension sized between 1 to 100 nanometers. They exhibit different properties than bulk materials due to greater surface area to volume ratio and quantum effects. Nanotechnology involves designing and engineering structures at the nanoscale to utilize these size-dependent properties. Nano materials find applications in industries such as electronics, energy, medicine, and more.