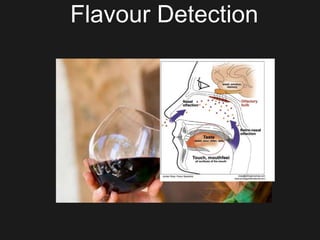
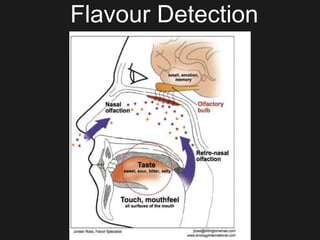
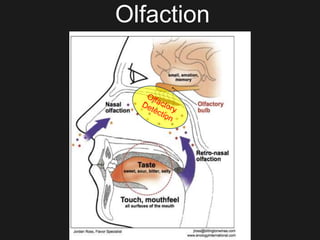
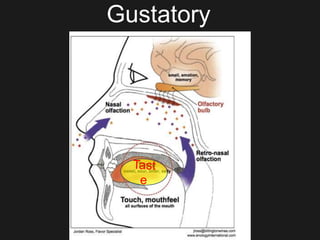
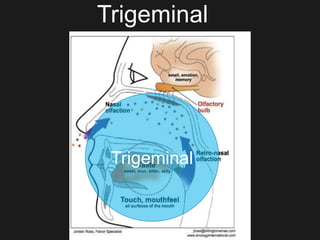
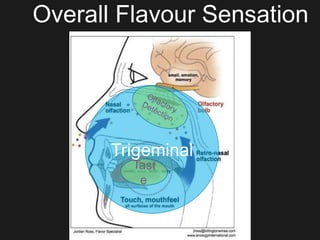
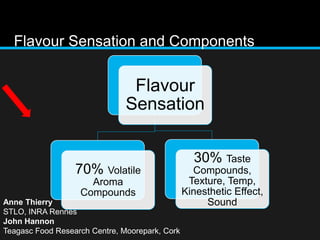
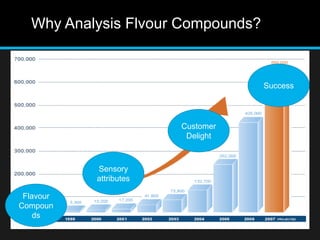
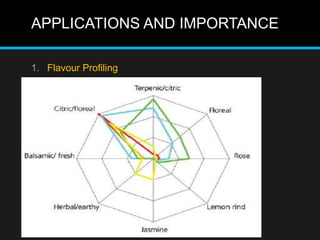
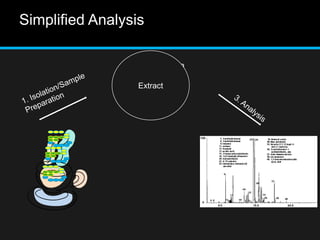
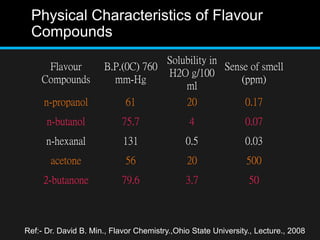
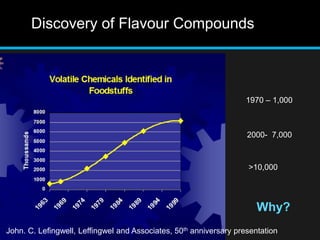
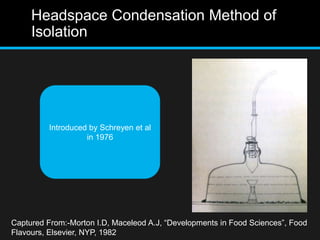
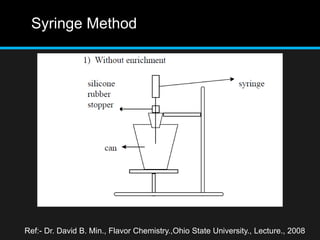
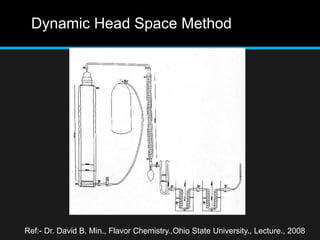
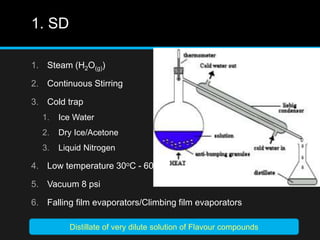
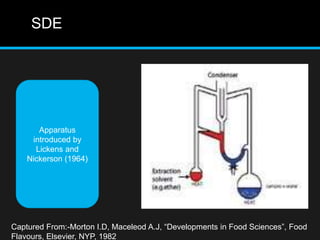
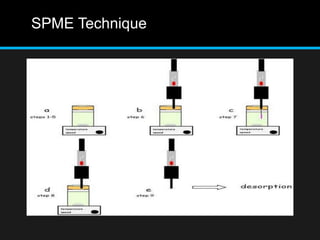
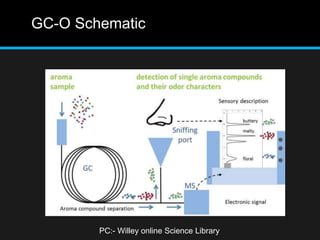
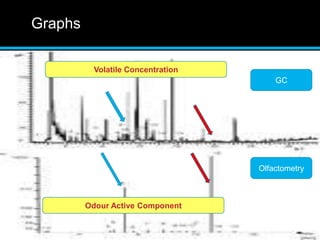
The document discusses techniques for analyzing food flavor compounds. It begins with an introduction to flavor, defining it as both a sensory sensation and the components that produce that sensation. It then covers various techniques for isolating volatile flavor compounds from foods, including headspace extraction methods like static headspace, dynamic headspace, and solid phase microextraction, as well as distillation and extraction techniques like steam distillation, solvent extraction, and simultaneous distillation-extraction. The document emphasizes that the choice of isolation technique depends on the objective and nature of the analyte compounds.