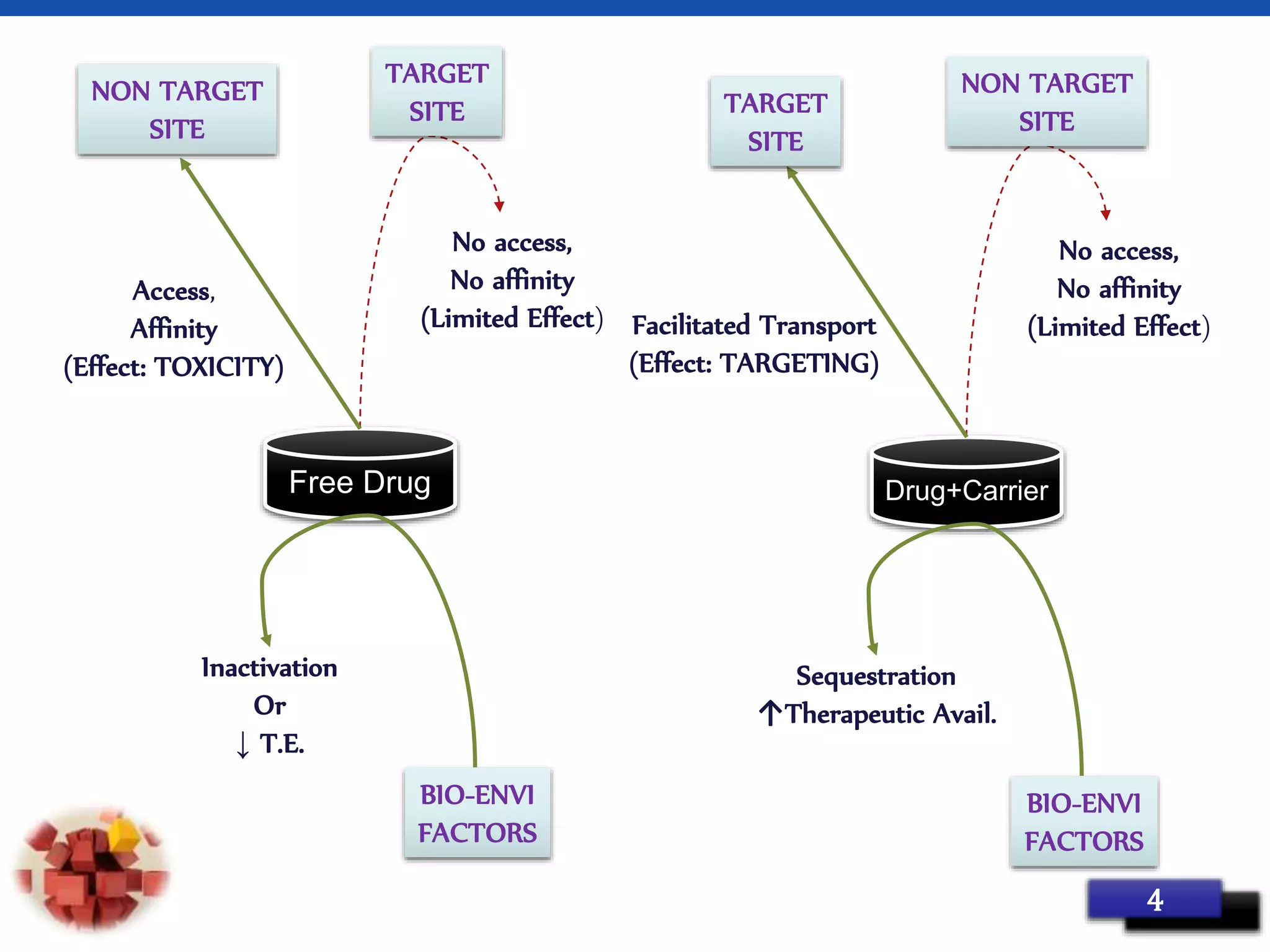
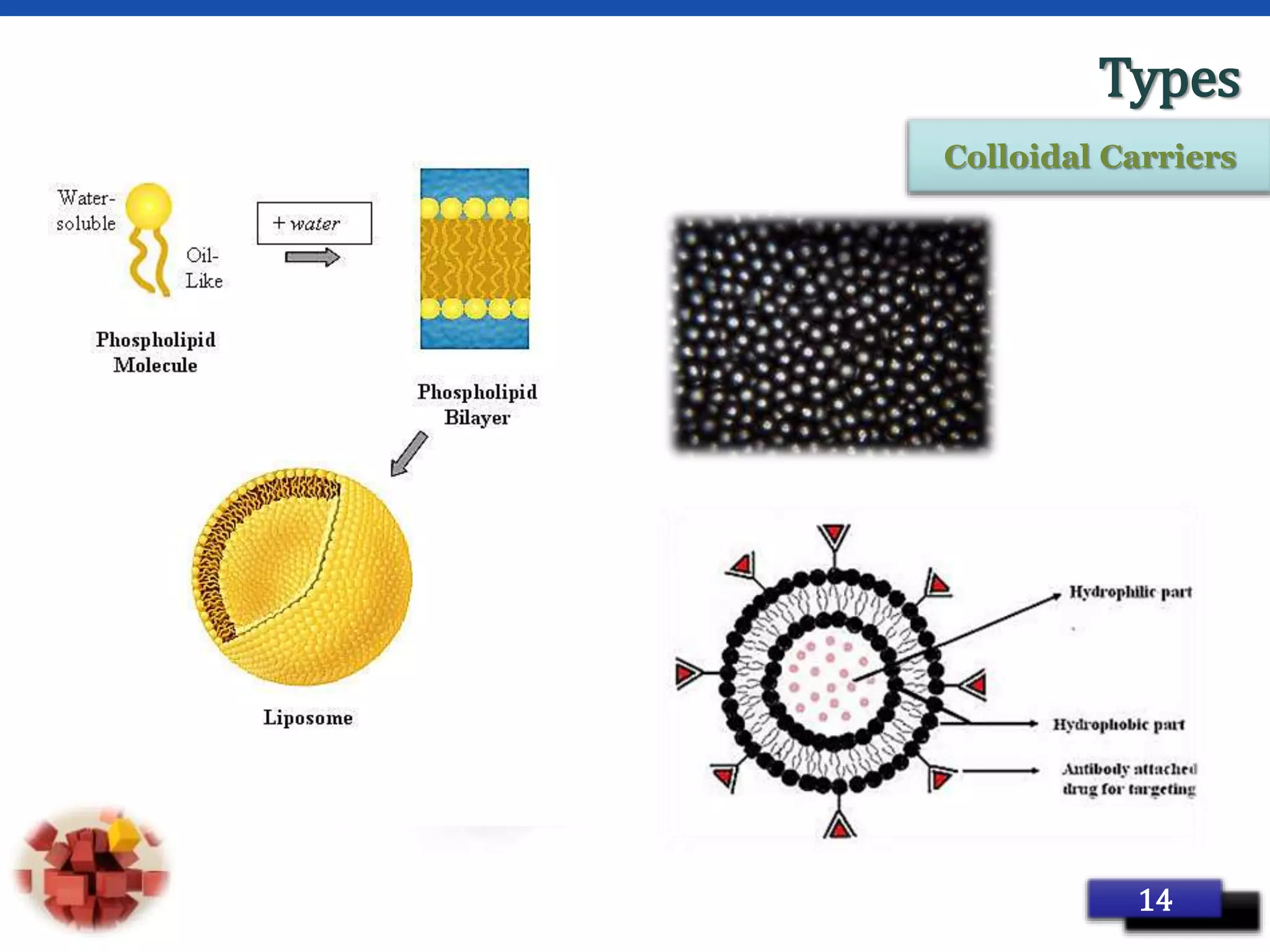
This document provides an overview of targeted drug delivery systems. It discusses the reasons for targeted delivery to increase therapeutic effects and reduce toxicity. The ideal properties of targeted delivery carriers and approaches are described. The document outlines different carrier types including vesicular, particulate, cellular, polymeric, and macromolecular systems. It discusses levels of targeting including passive, active, dual and combination approaches. Active targeting can be achieved through ligand-mediated or physical approaches. The document provides examples to illustrate different targeting strategies and carrier types. In summary, it comprehensively reviews concepts and components of targeted drug delivery systems.