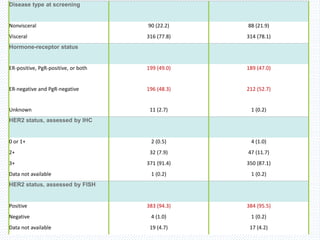
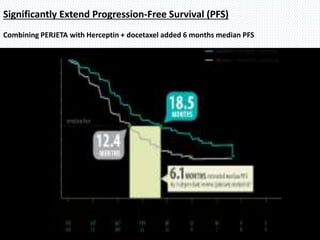
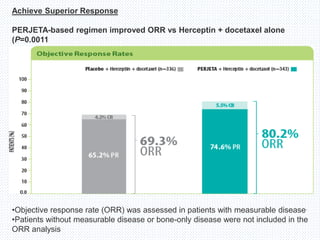
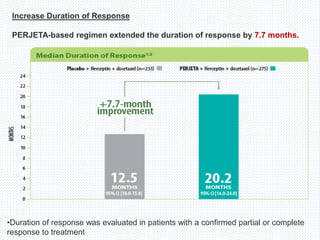
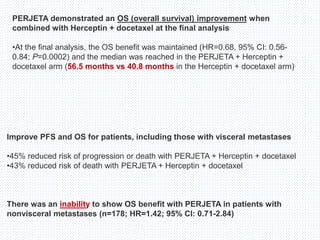
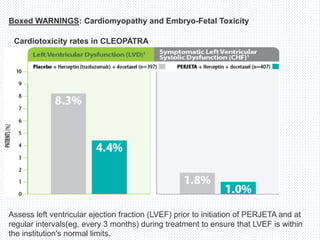
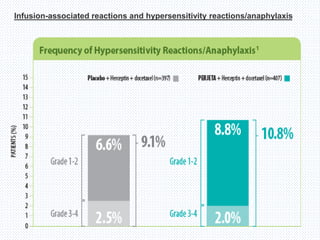
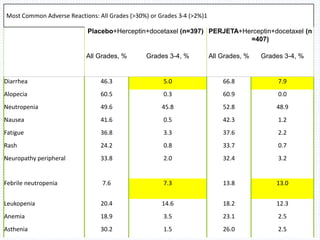
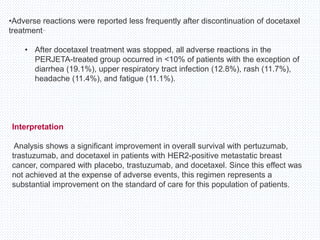
The CLEOPATRA trial was a phase III randomized controlled trial that compared the efficacy and safety of pertuzumab, trastuzumab, and docetaxel versus placebo, trastuzumab, and docetaxel in patients with previously untreated HER2-positive metastatic breast cancer. The study found that adding pertuzumab to trastuzumab and docetaxel significantly extended progression-free survival by 6 months and improved overall response rates compared to the placebo group. Overall survival was also improved with the pertuzumab regimen. While rates of adverse events were similar between the groups, the pertuzumab regimen represented a substantial improvement over the standard of care.

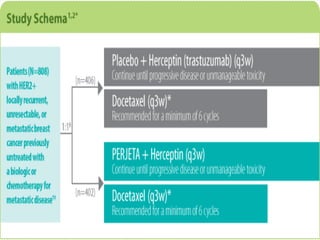
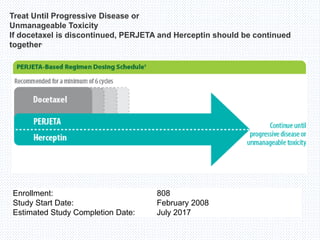
![Study design
•CLEOPATRA was a multicenter, randomized, double-blind, placebo-controlled phase
III trial
•Primary end point
Progression-free survival (PFS; as assessed by an independent review facility [IRF]),
defined as time from randomization to documented progressive disease as
determined by RECIST, or death.
•Secondary end points
Overall survival (OS)
Objective response rate (ORR)
Duration of response (DoR)
Safety](https://image.slidesharecdn.com/cleopatra-150819164531-lva1-app6891/85/Cleopatra-trial-2-320.jpg)

![Baseline Characteristics2
Placebo + Herceptin +
docetaxel
n=406, n (%)
PERJETA + Herceptin +
docetaxel
n=402, n (%)
Female sex 404 (99.5) 402 (100)
Age — years
Median [range] 54.0 [27-89] 54.0 [22-82]
Race or ethnic group*
Asian 133 (32.8) 128 (31.8)
Black 20 (4.9) 10 (2.5)
White 235 (57.9) 245 (60.9)
Other 18 (4.4) 19 (4.7)
Region
Asia 128 (31.5) 125 (31.1)
Europe 152 (37.4) 154 (38.3)
North America 68 (16.7) 67 (16.7)
South America 58 (14.3) 56 (13.9)](https://image.slidesharecdn.com/cleopatra-150819164531-lva1-app6891/85/Cleopatra-trial-6-320.jpg)