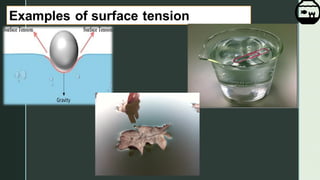
Surface tension is a property of liquids that allows their surfaces to resist external forces. It is caused by cohesive forces between liquid molecules at the surface that make the surface behave like an elastic membrane. Examples that demonstrate surface tension include insects walking on water and small droplets forming spherical shapes. Surface tension can be calculated using the formula F=S*L, where F is the applied force, S is the surface tension, and L is the length of an imaginary line on the surface. Surface tension is affected by temperature and impurities, decreasing with increasing temperature and increasing or decreasing depending on whether impurities are soluble or not.



![FORMULA
The force acting on the line is proportional to the length of the line.
Where, l= length of imaginary line
F= total force apply on line
Then,
F L F= S.L
S = 1 F
2 L UNIT is N/m
Dimension is [MT-2]
Or Surface tension,S = force
Length](https://image.slidesharecdn.com/surfacetension-230901052351-1d159a14/85/Surface-tension-pdf-5-320.jpg)




