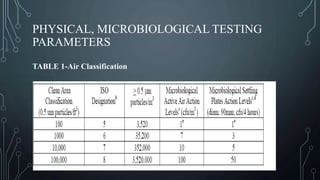
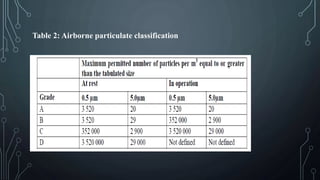
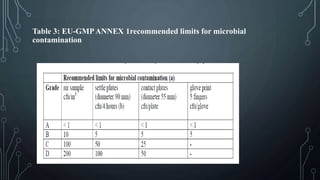
This document discusses validation of critical utilities in the pharmaceutical industry. It covers validation of various systems including purified water systems, compressed air systems, HVAC systems, and clean steam systems. It describes the FDA requirements for validation which includes installation qualification, operational qualification and performance qualification. It provides specifications for purified water and discusses environmental monitoring programs for validated utilities.