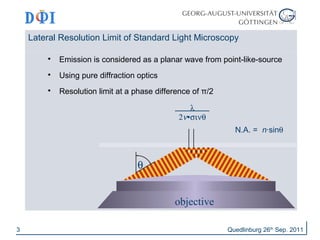
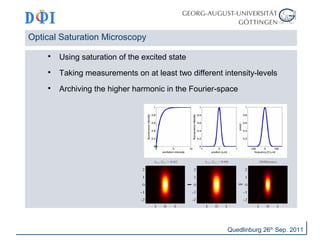
The document discusses advancements in imaging techniques such as Image Scanning Microscopy (ISM) and Superresolution Optical Fluctuation Imaging (SOFI) presented by Dirk Hähnel and Jörg Enderlein during a summer school in Quedlinburg, 2011. ISM improves resolution compared to conventional confocal imaging by replacing point-detectors and enhancing 3D imaging capabilities, while SOFI offers simple implementation and improved background elimination for blinking probes. Both techniques aim to enhance imaging quality in biological contexts, with potential resolution increases of up to a factor of three.