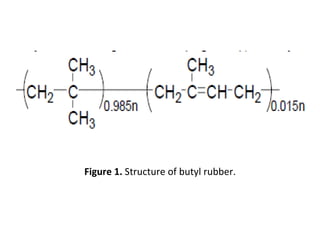
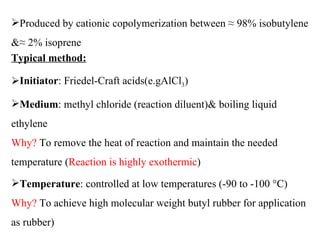
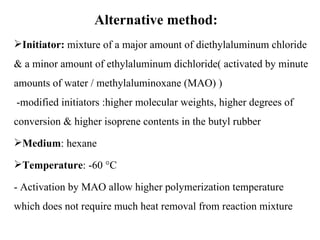
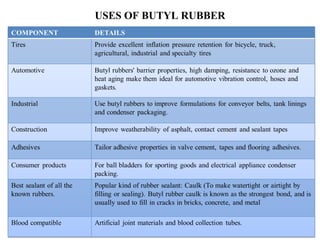
Butyl rubber is a synthetic rubber that is a copolymer of isobutylene and isoprene. It is produced via cationic copolymerization of 98% isobutylene and 2% isoprene using an initiator like Friedel-Craft acids at low temperatures between -90 to -100°C. Butyl rubber has excellent impermeability and flexibility due to its structure with long polyisobutylene segments separated by double bonds from the isoprene units. It has many desirable properties including low permeability, vibration damping, resistance to aging and weathering, fast cure rates, and being the best sealant of all known rubbers. These properties make butyl rubber useful in various applications