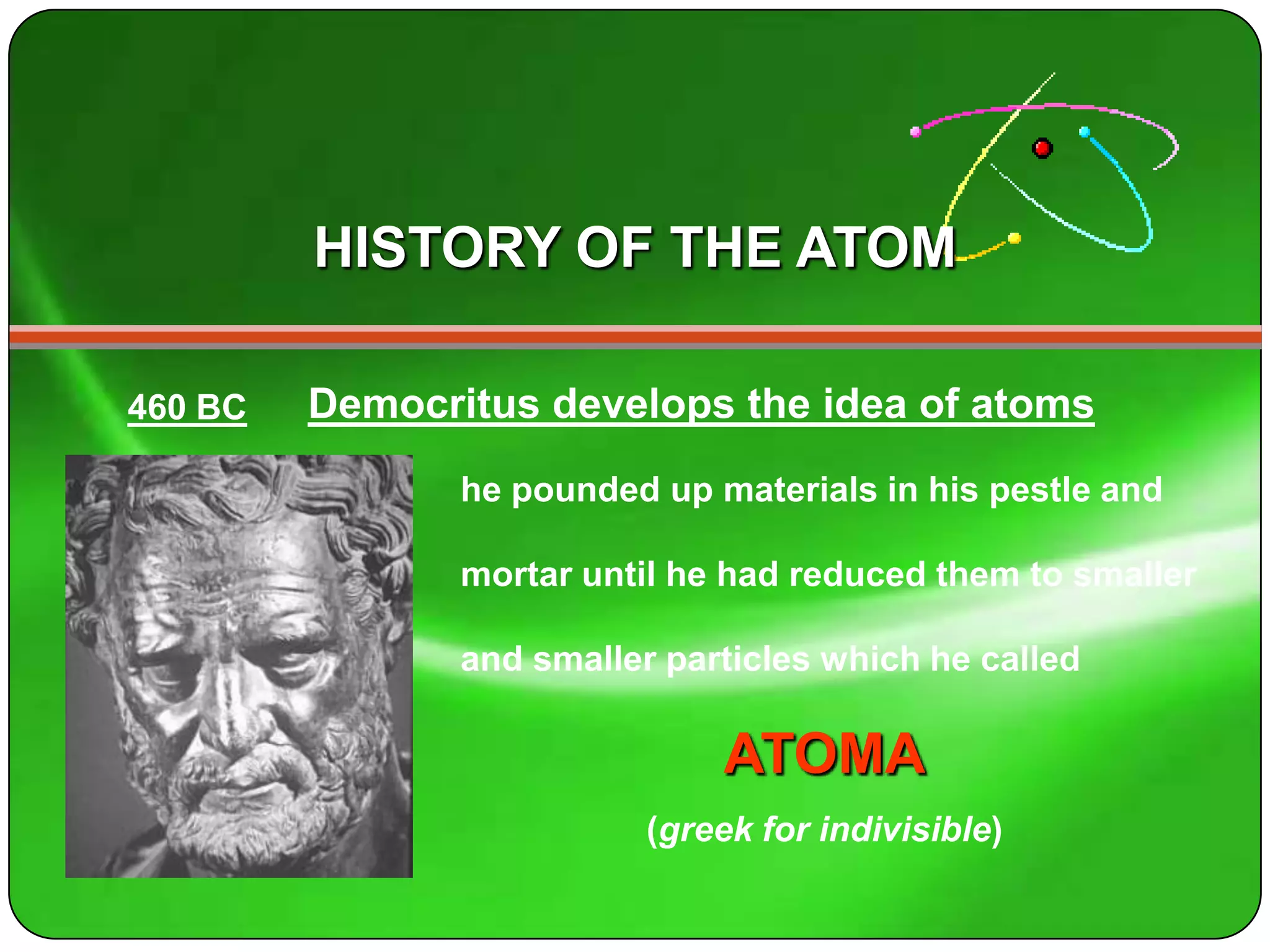
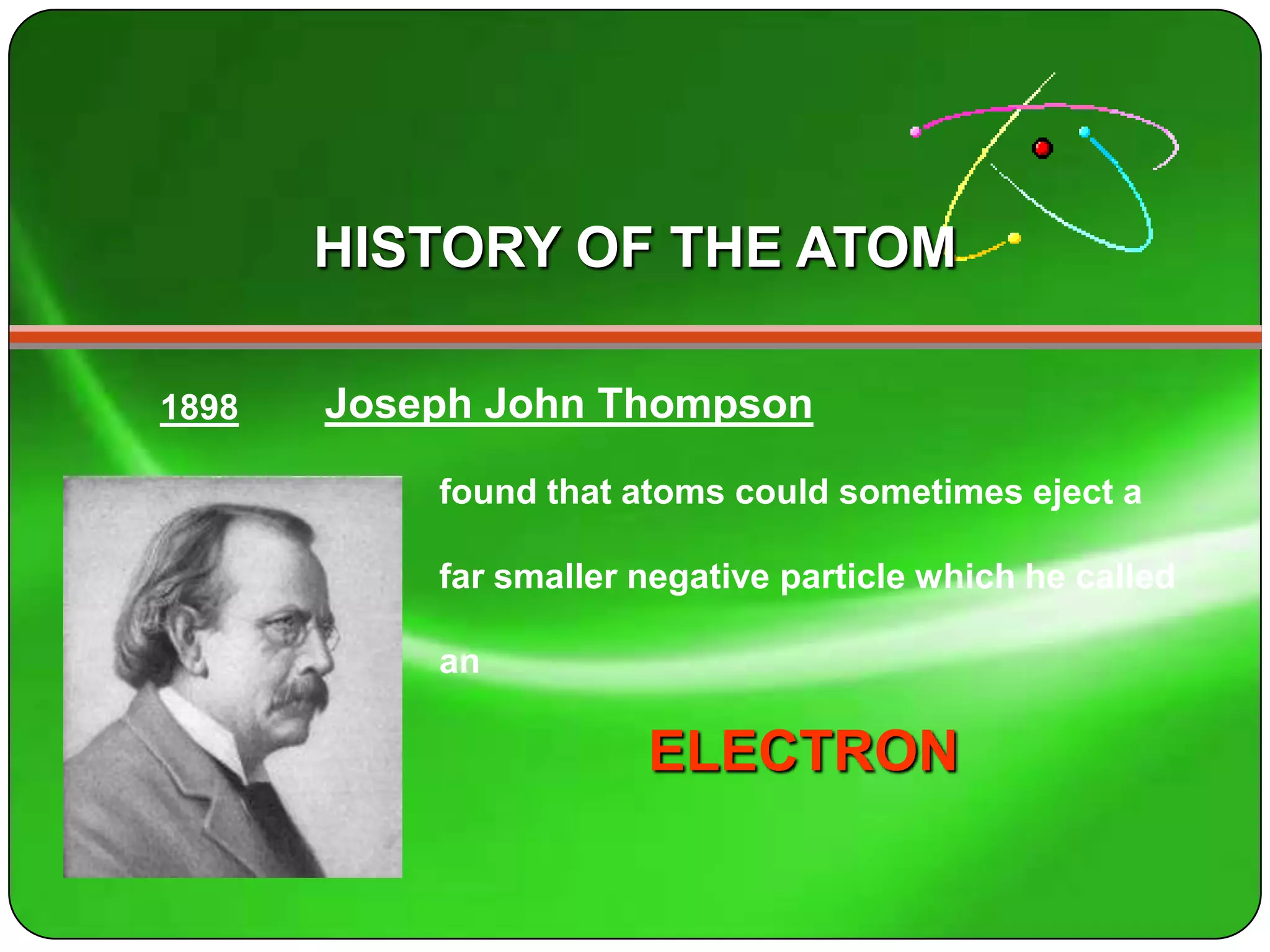
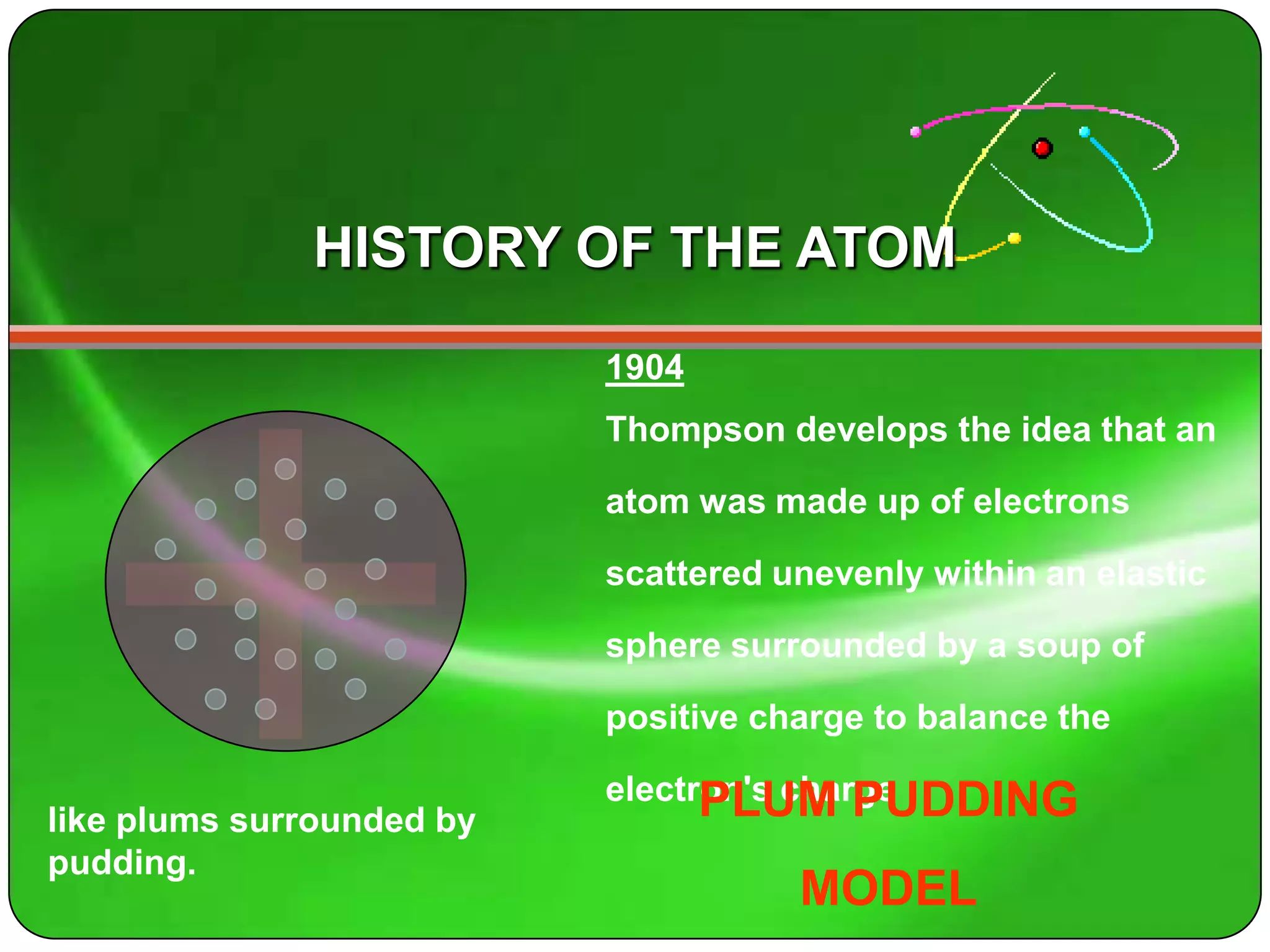
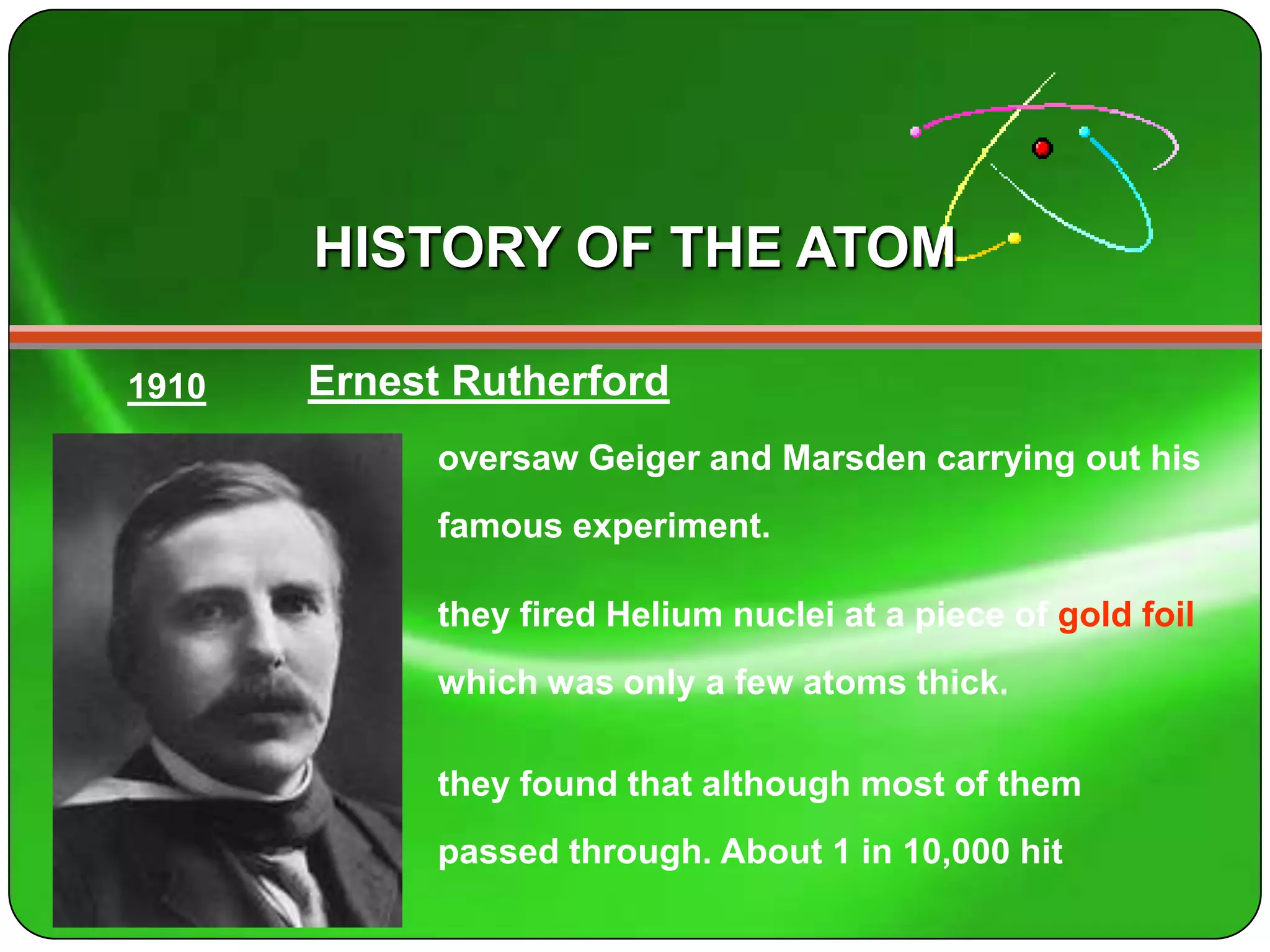
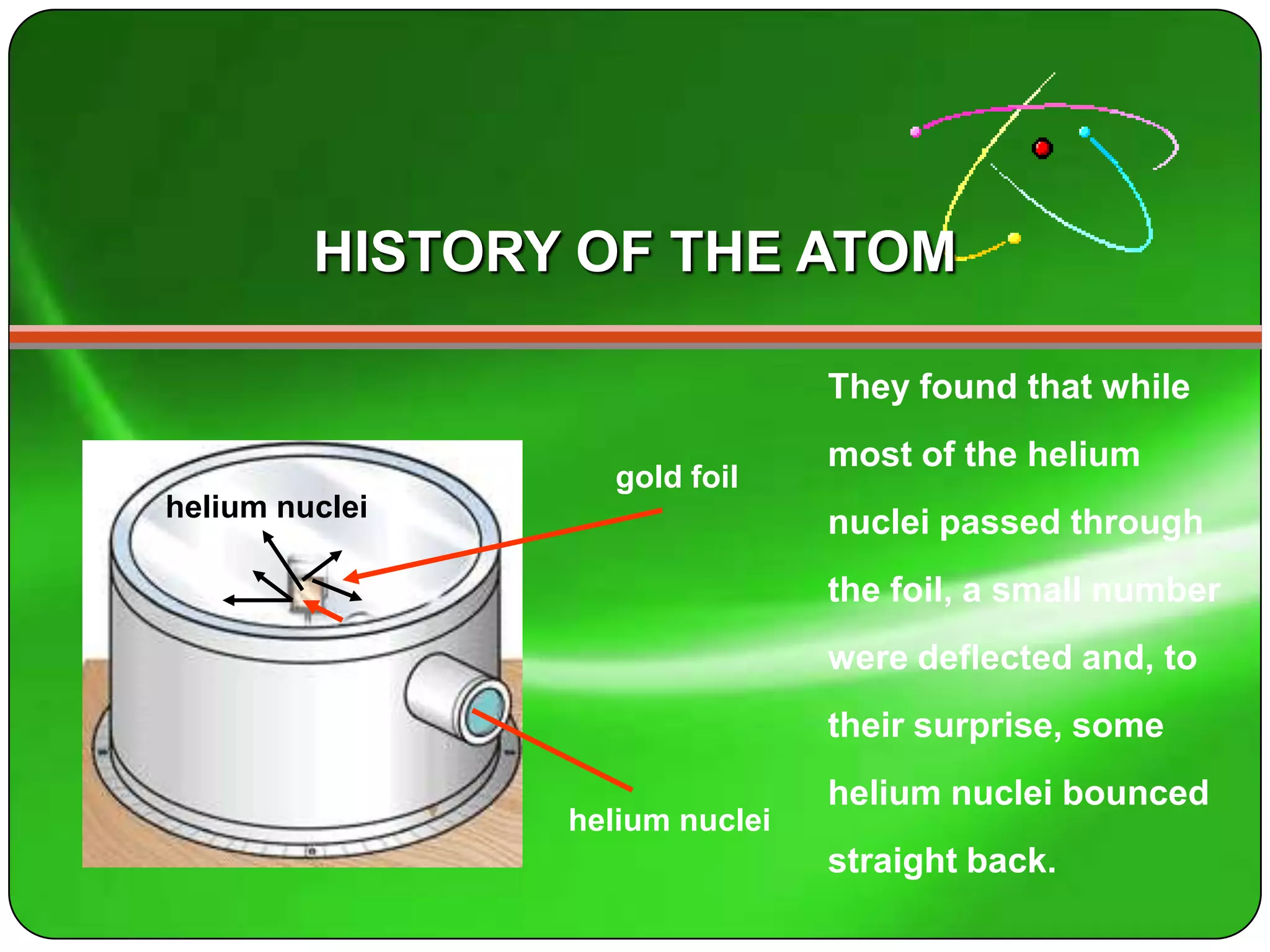
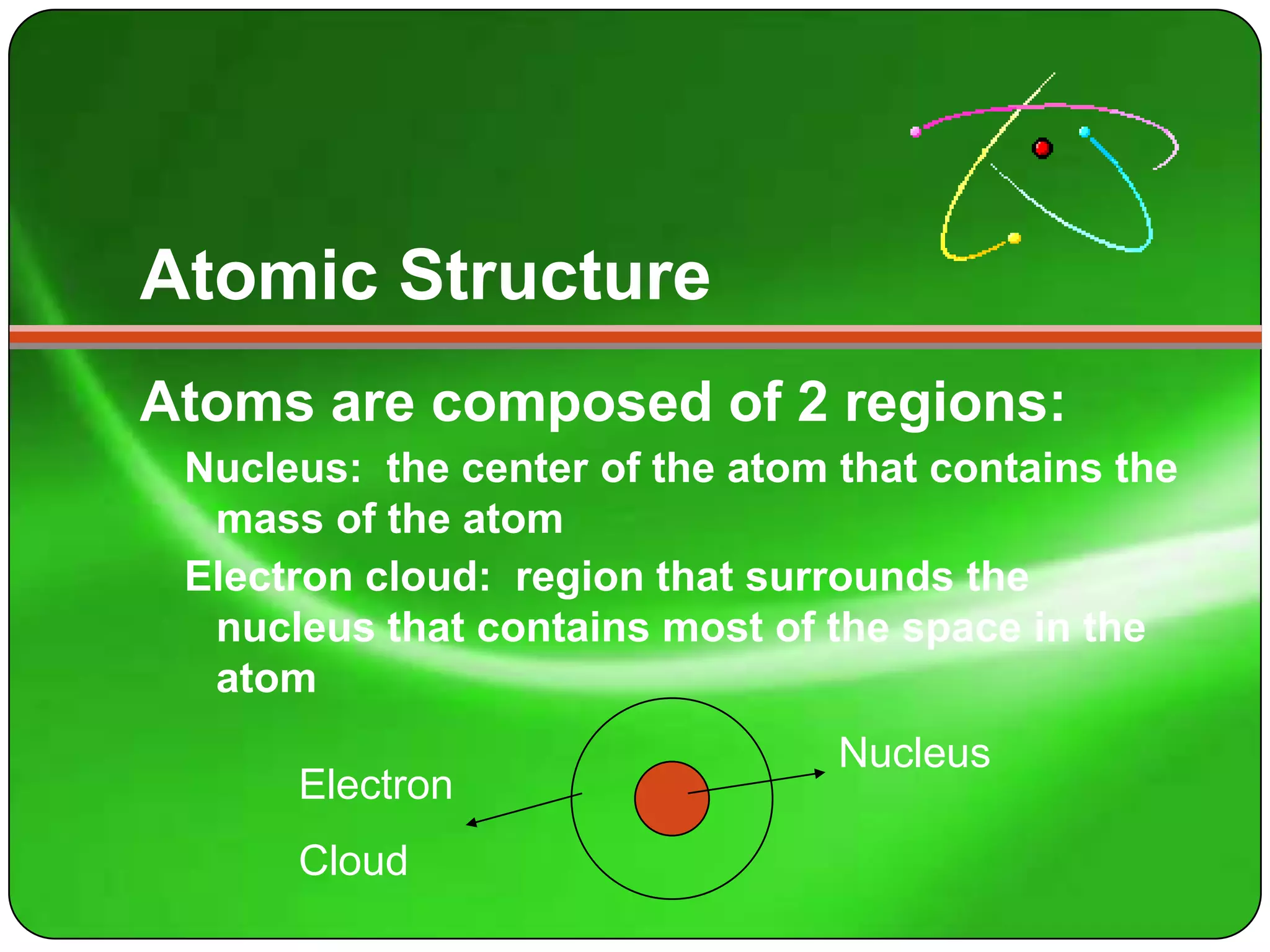
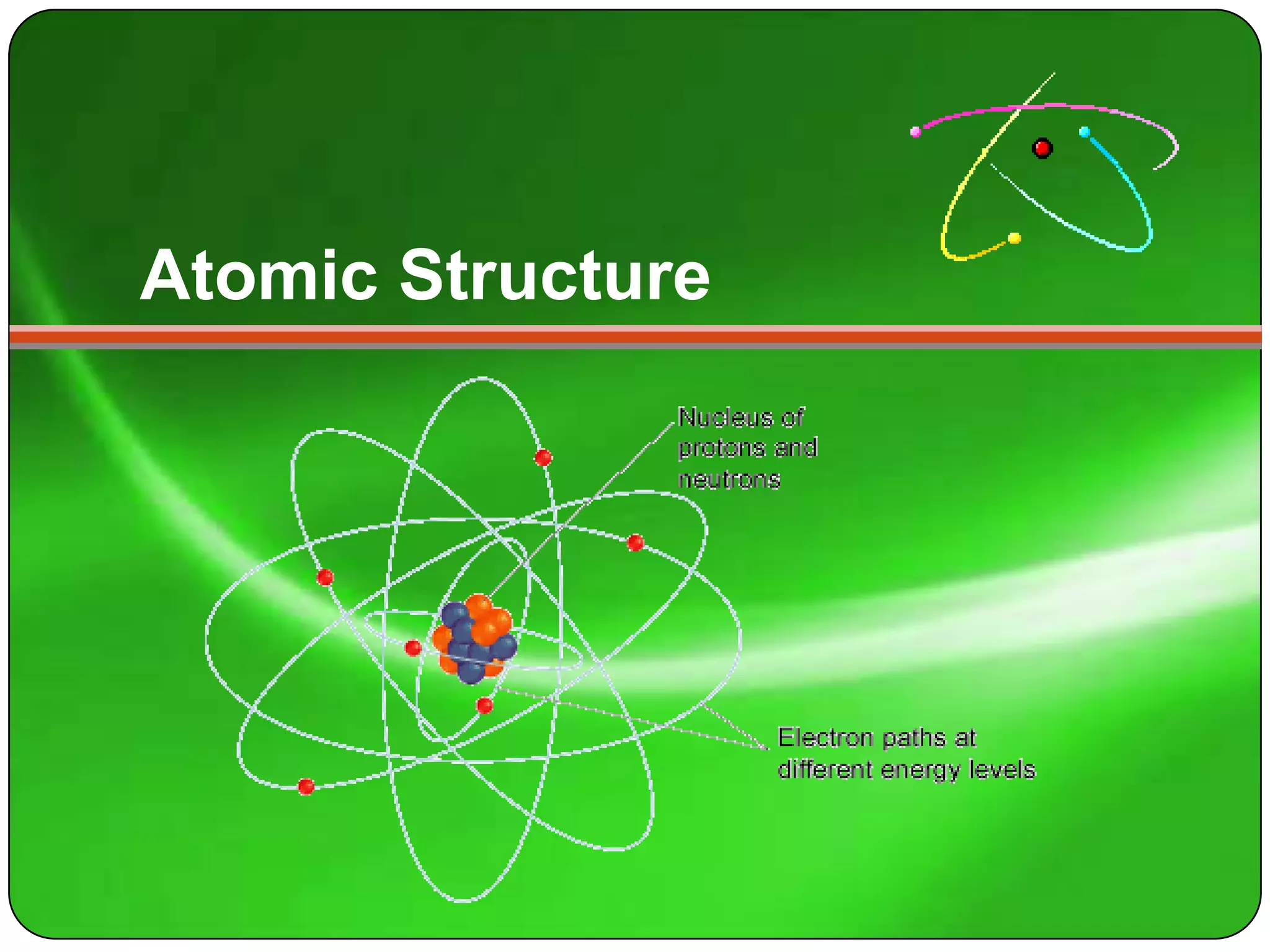
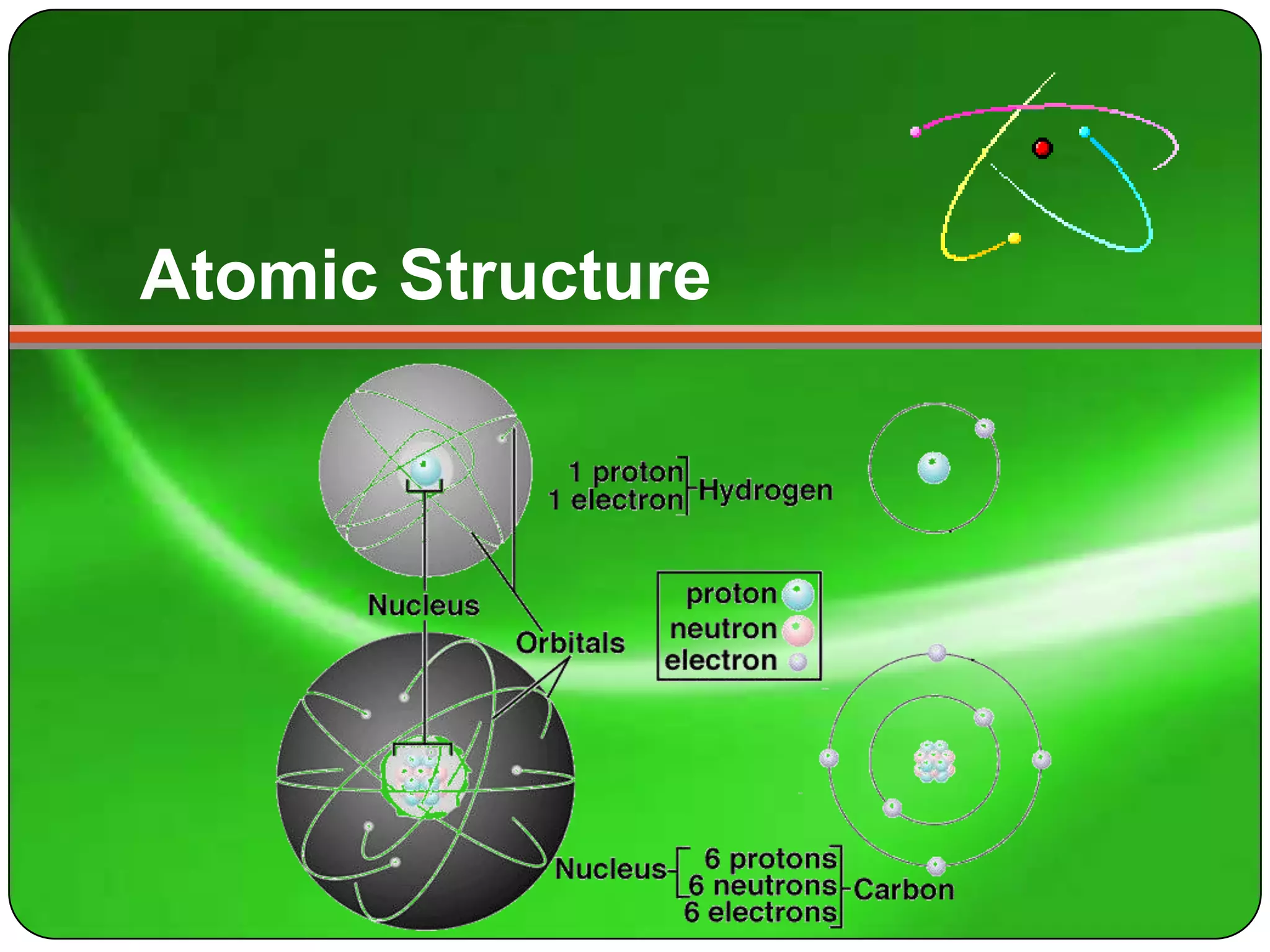
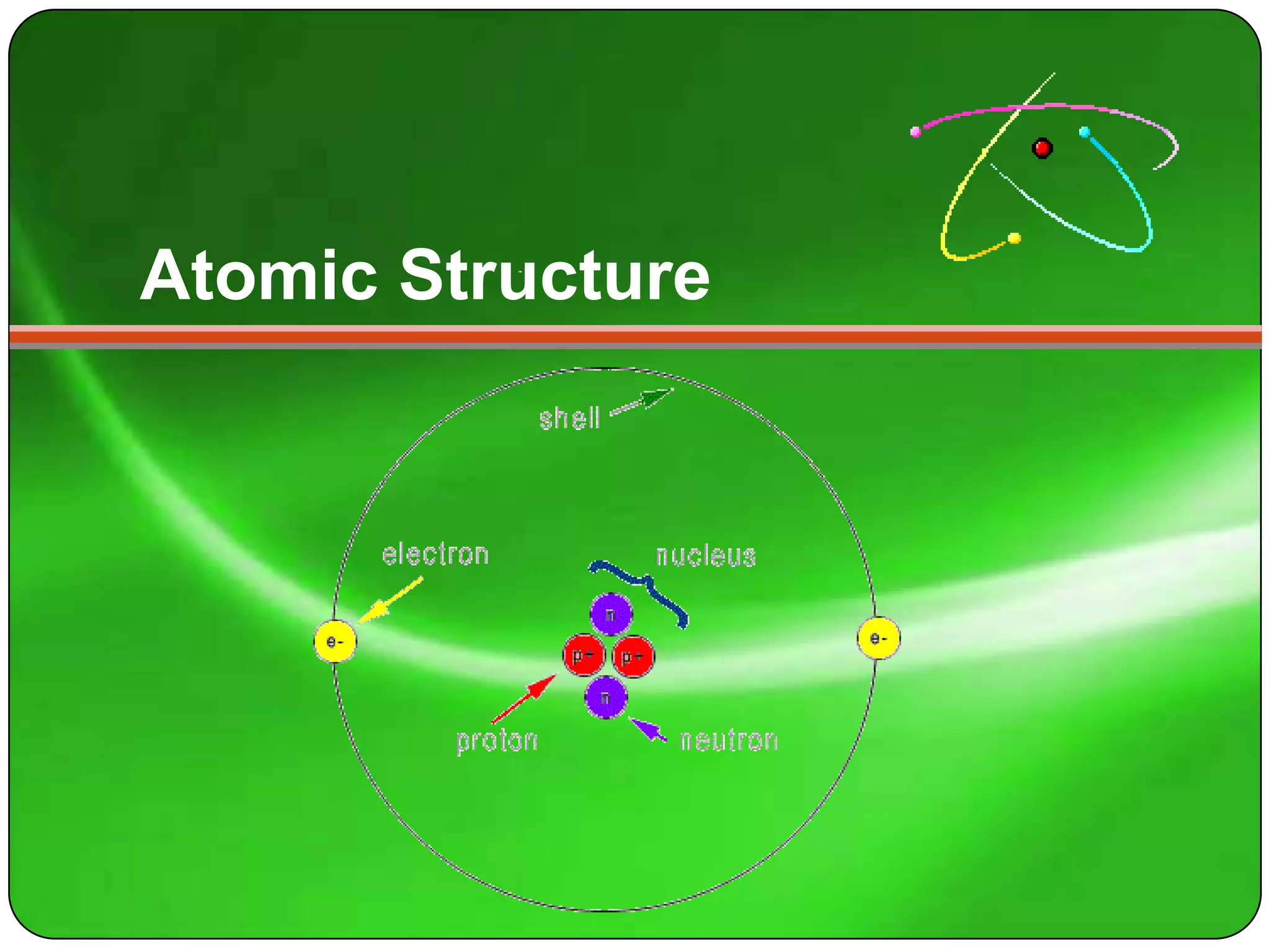
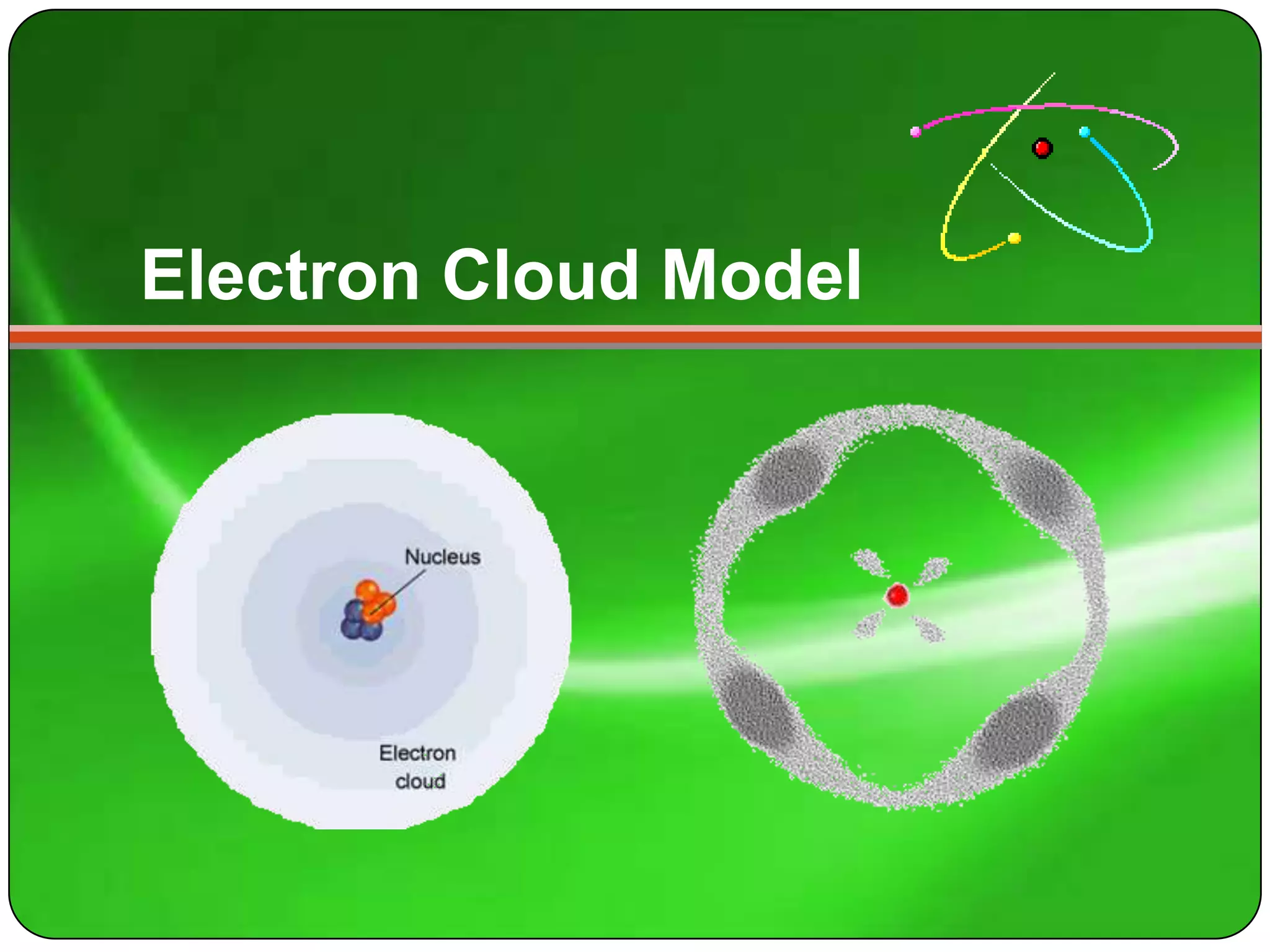
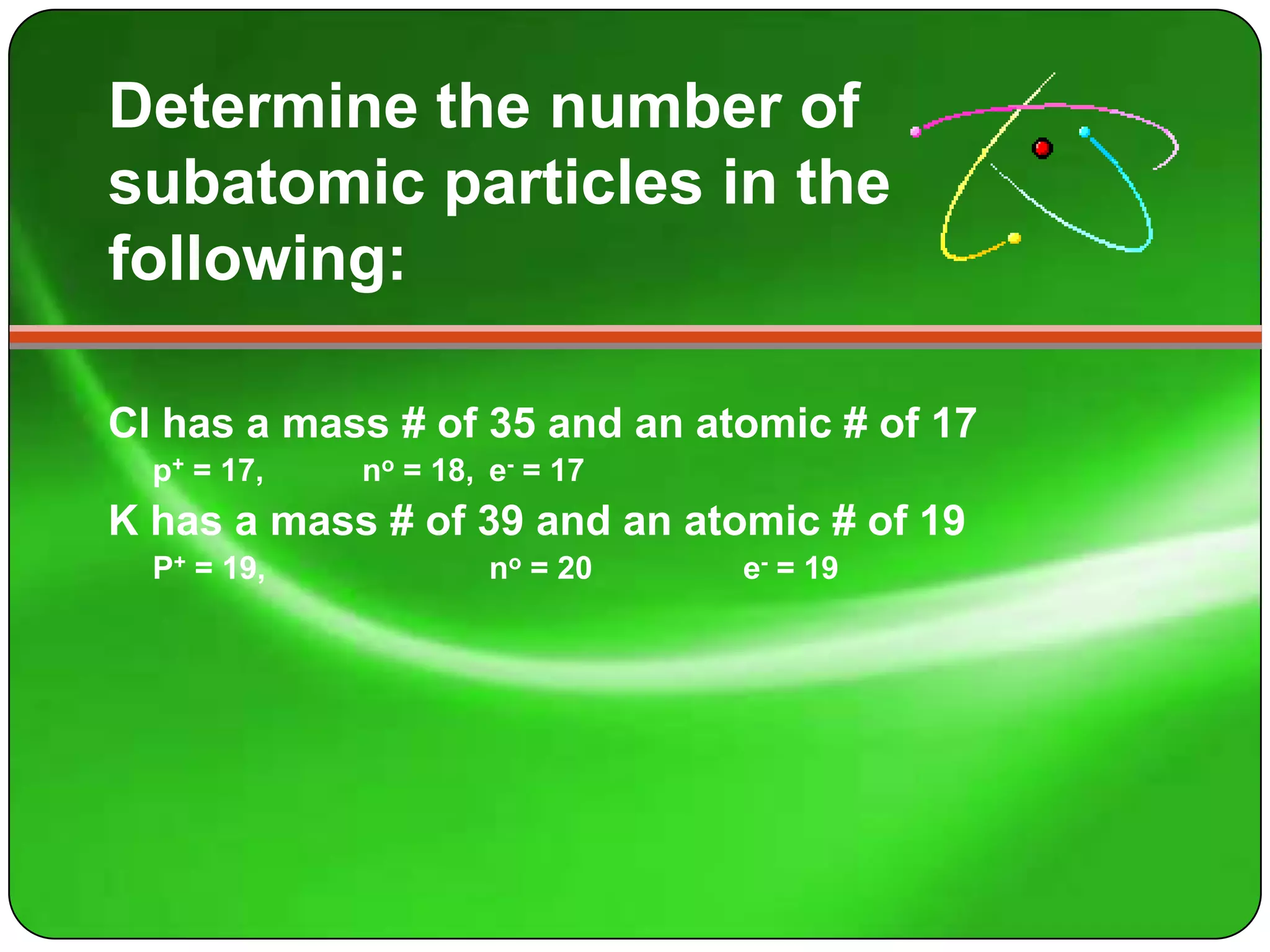
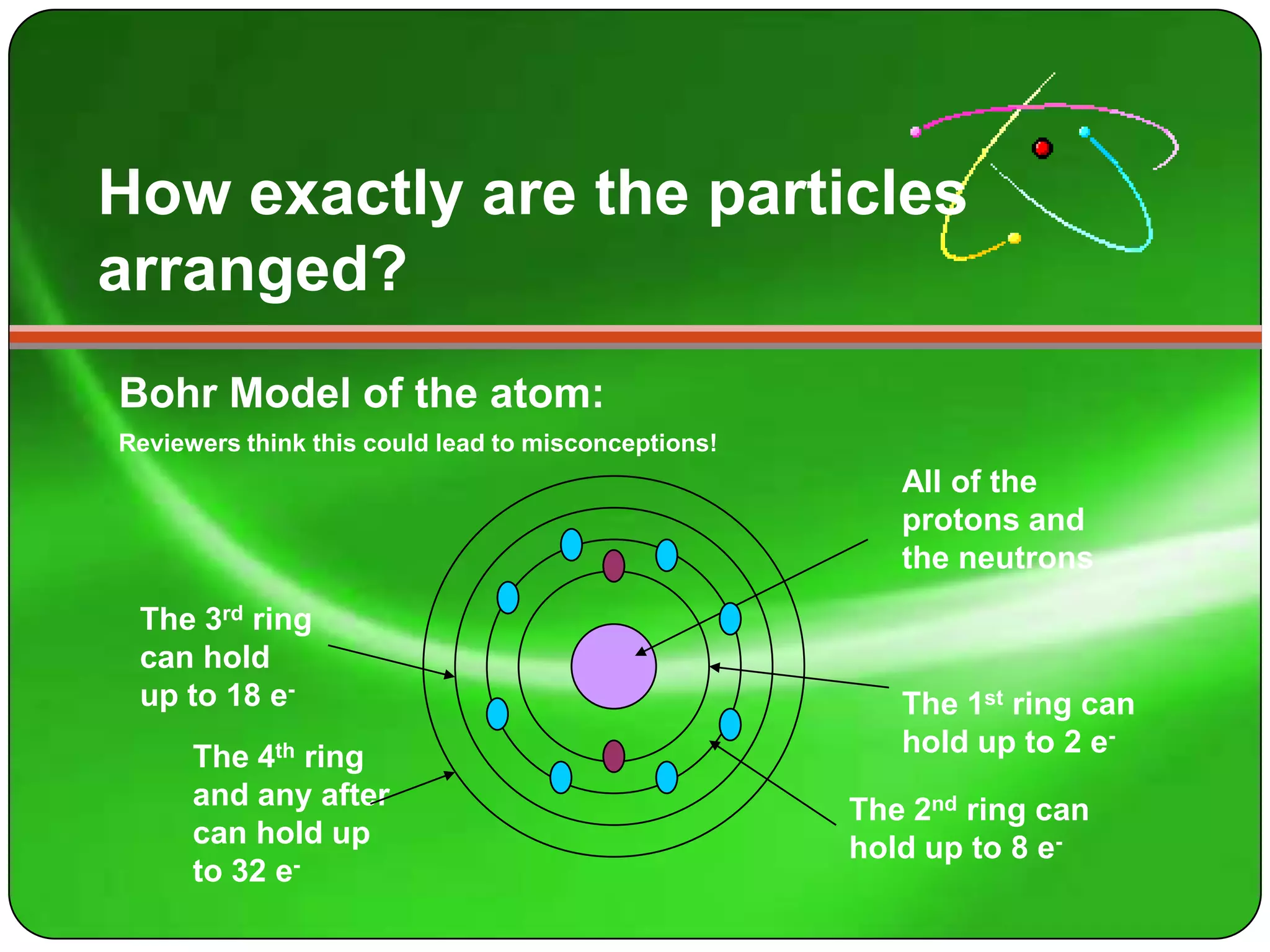
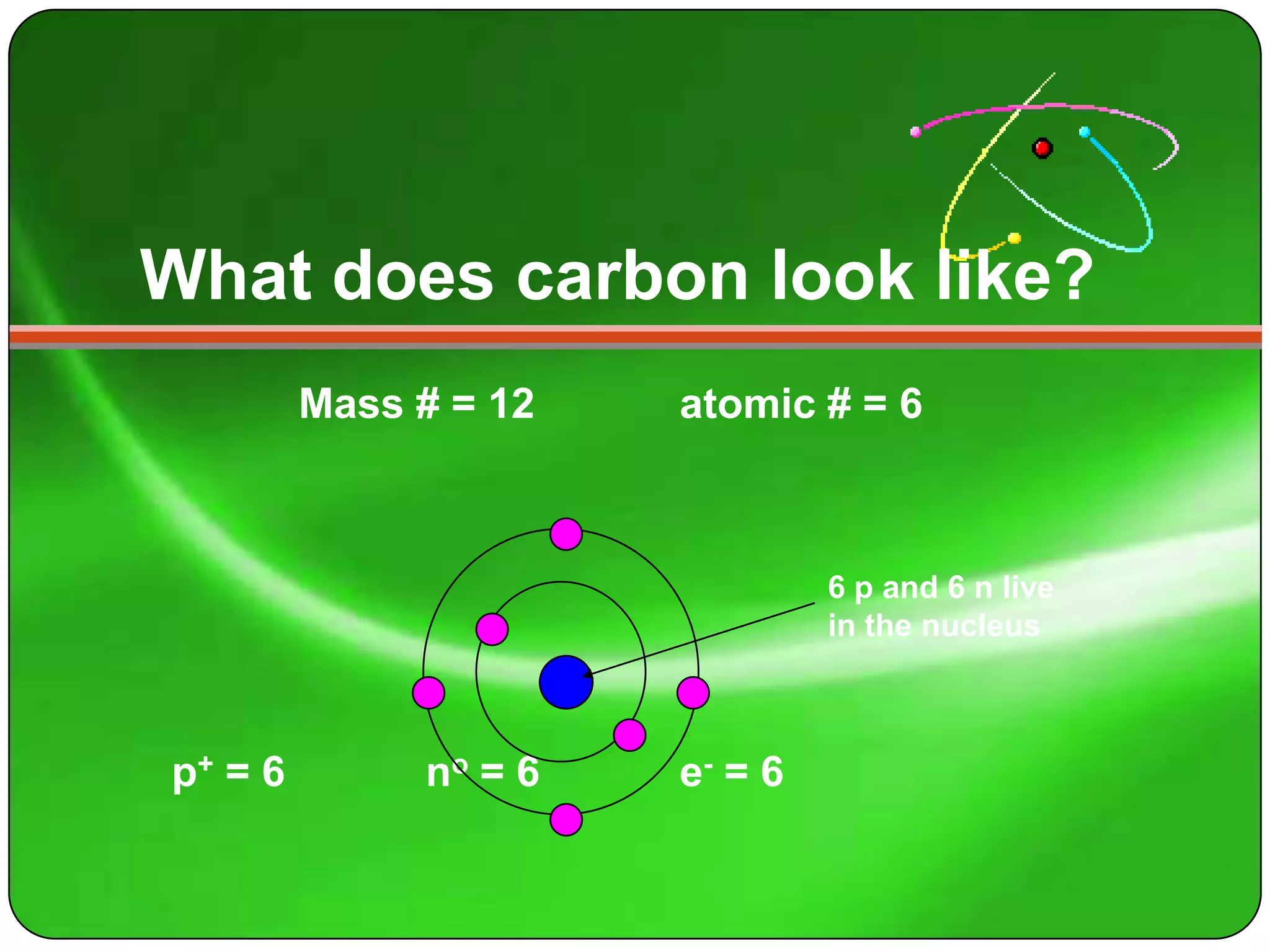
Here is a summary of the key points about carbon's atomic structure based on the information provided:
- Carbon has 6 protons and 6 neutrons in its nucleus.
- Its mass number is 12 and atomic number is 6.
- The number of protons equals the atomic number of 6.
- The number of neutrons is the mass number (12) minus the atomic number (6), which is 6.
- The number of electrons orbiting the nucleus equals the number of protons, which is 6.
So in summary, carbon has 6 protons and 6 neutrons in its nucleus, and 6 electrons orbiting the nucleus.