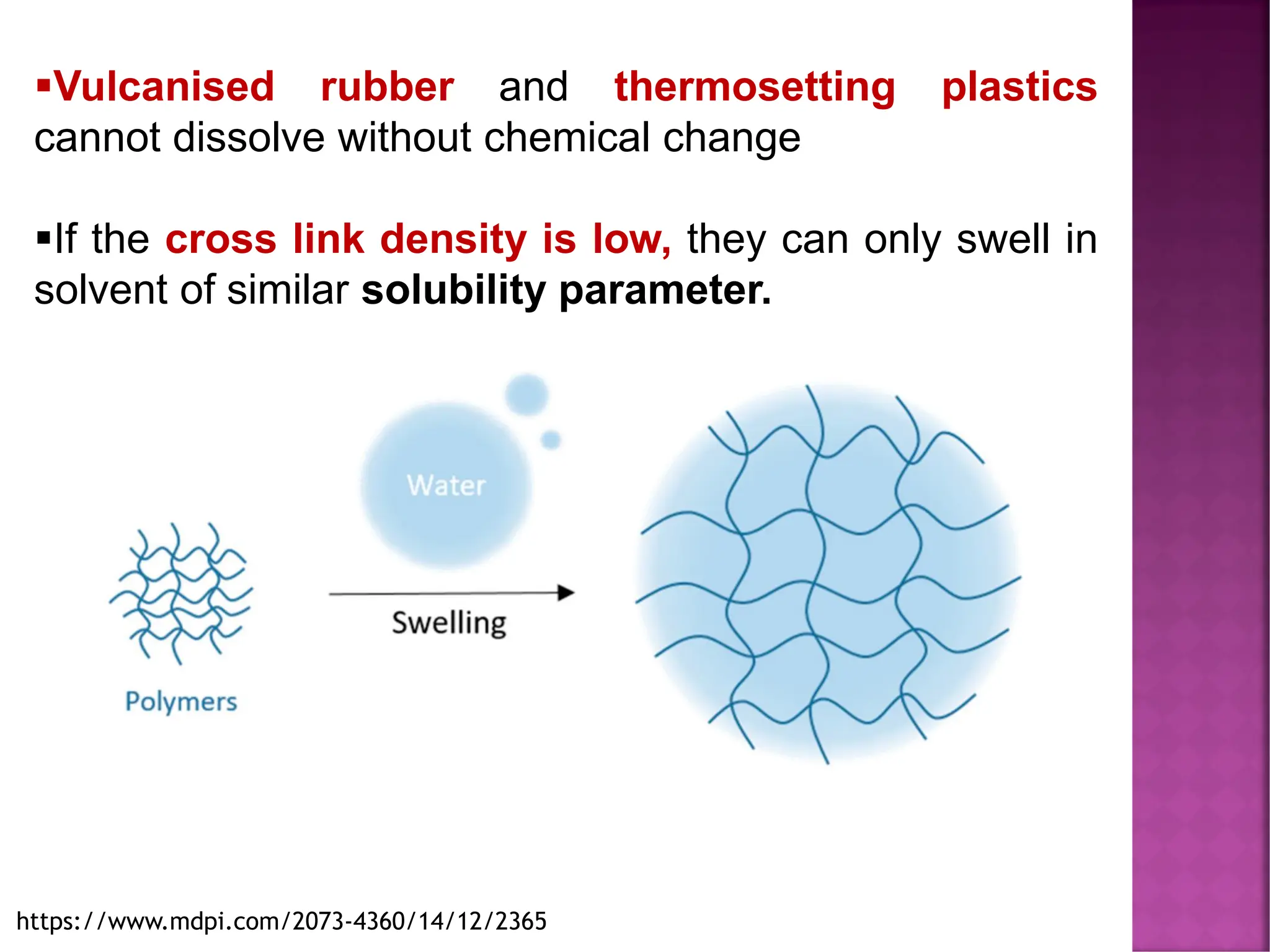
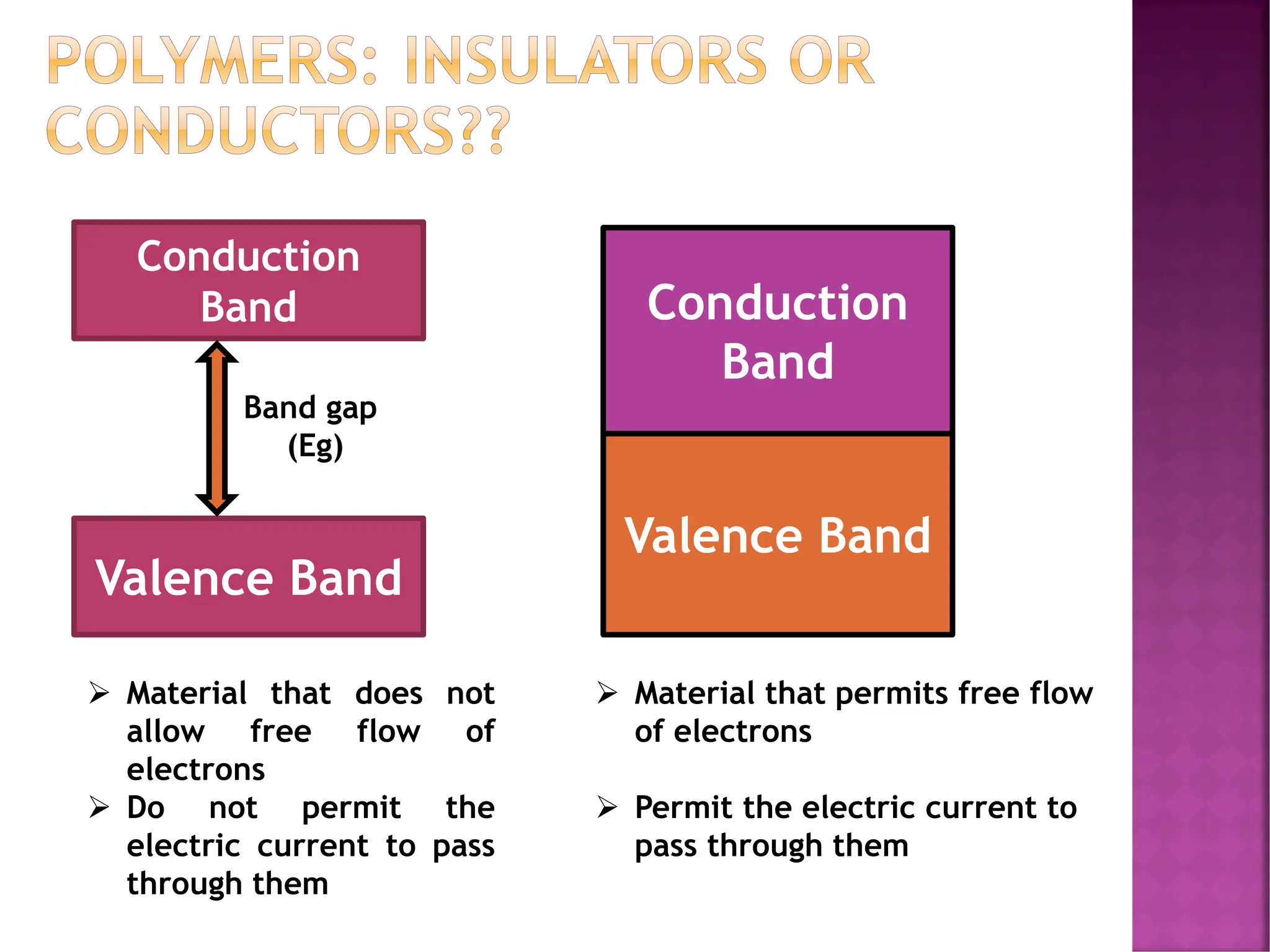
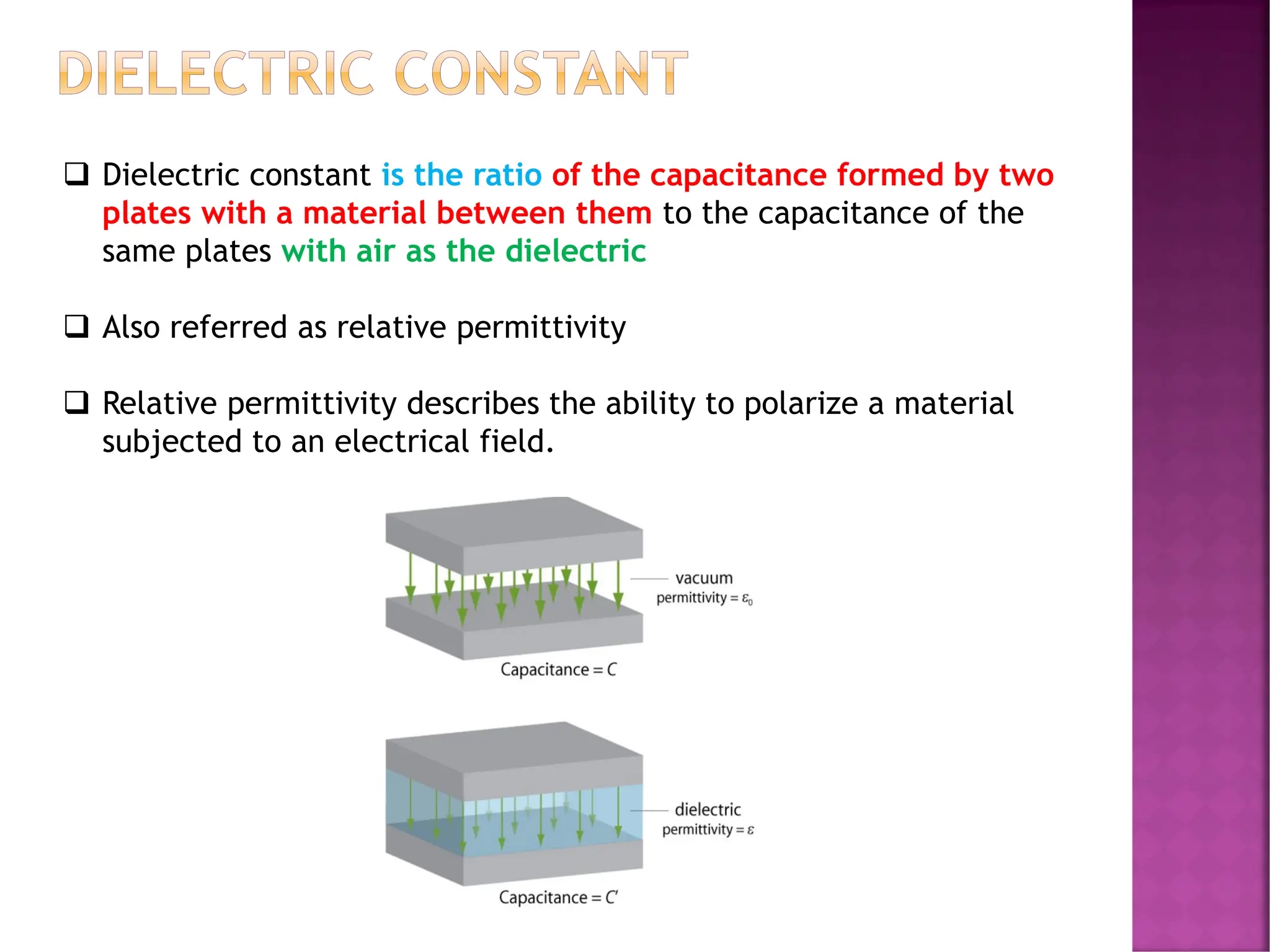
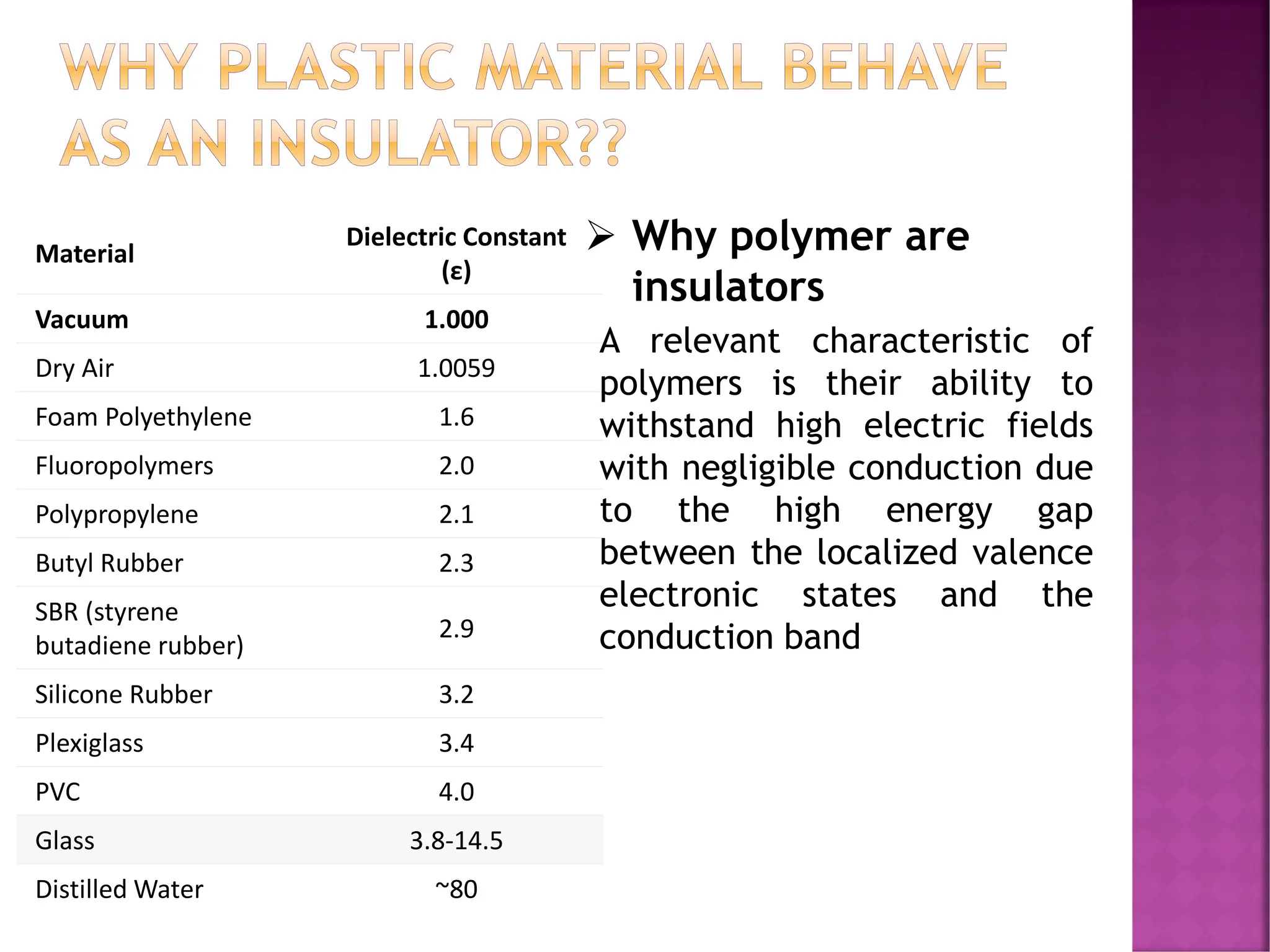
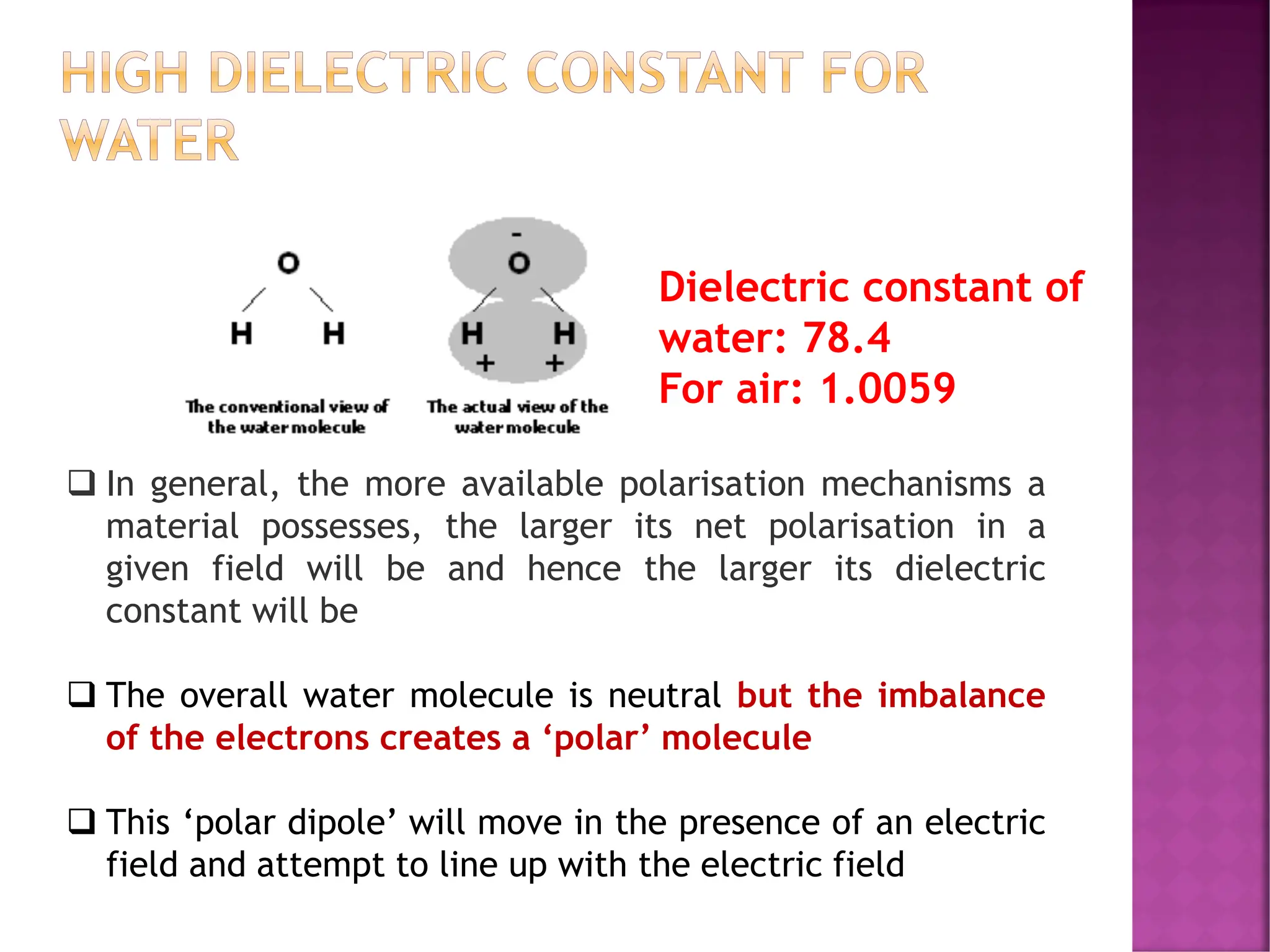
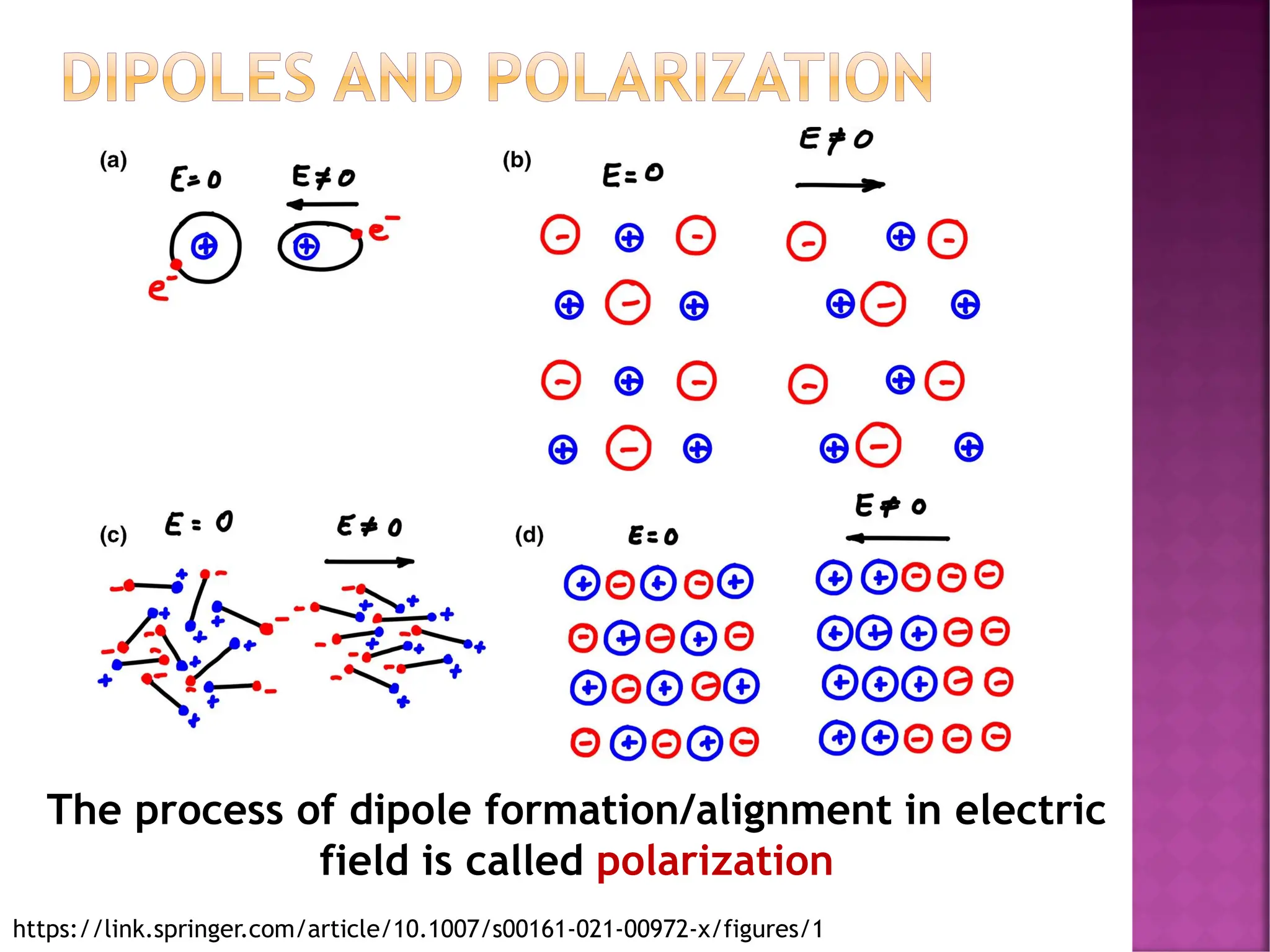
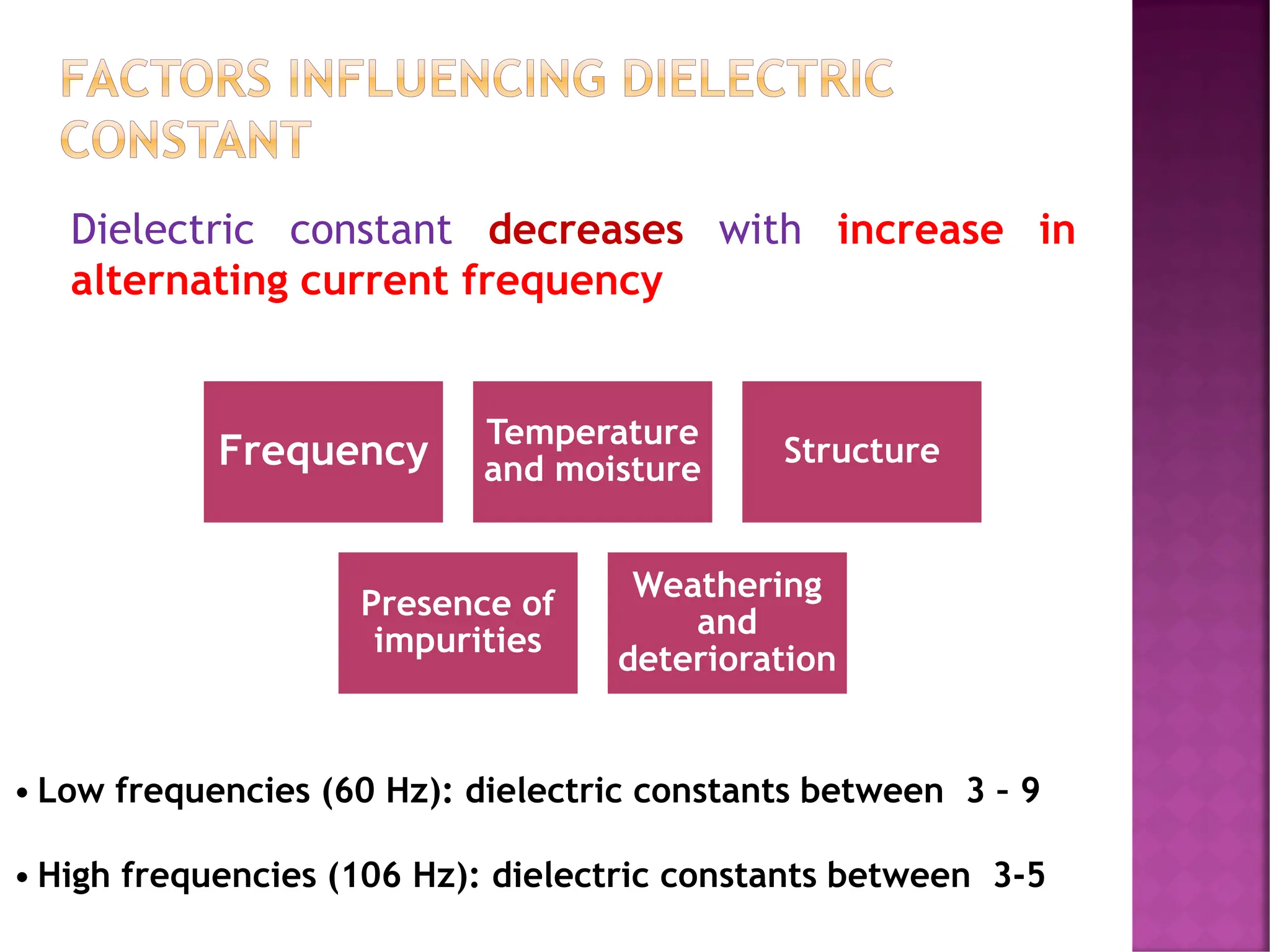
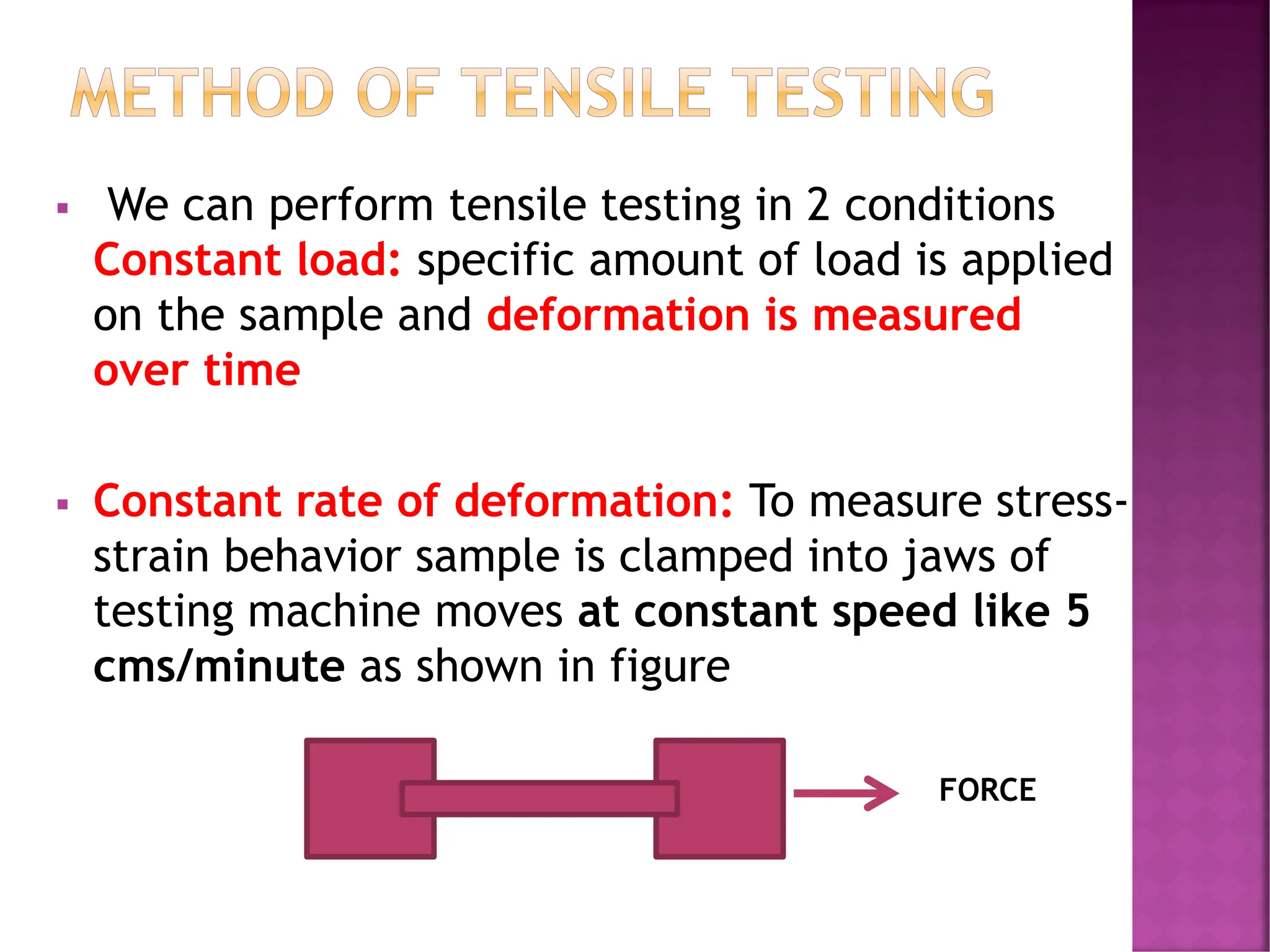
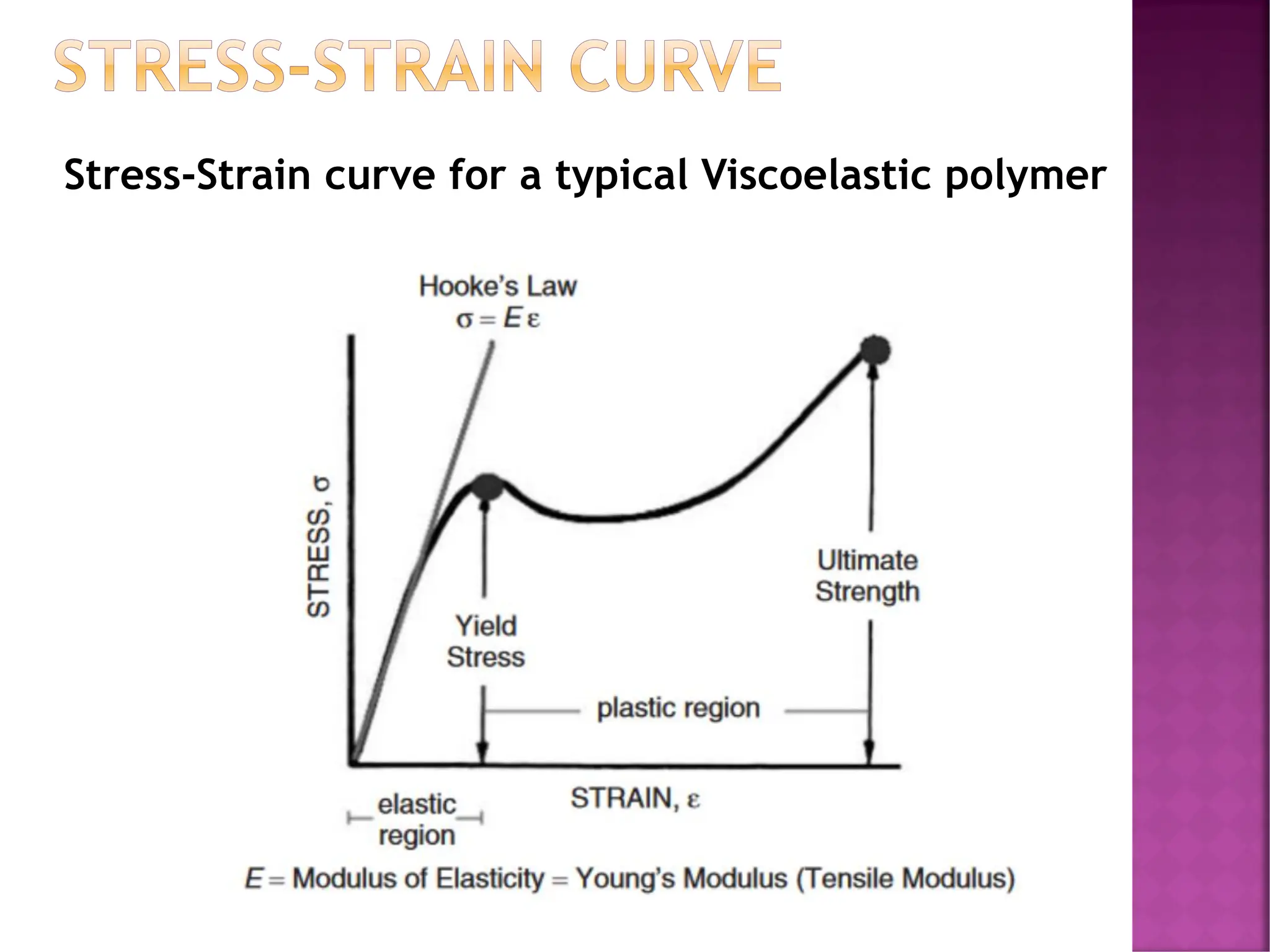
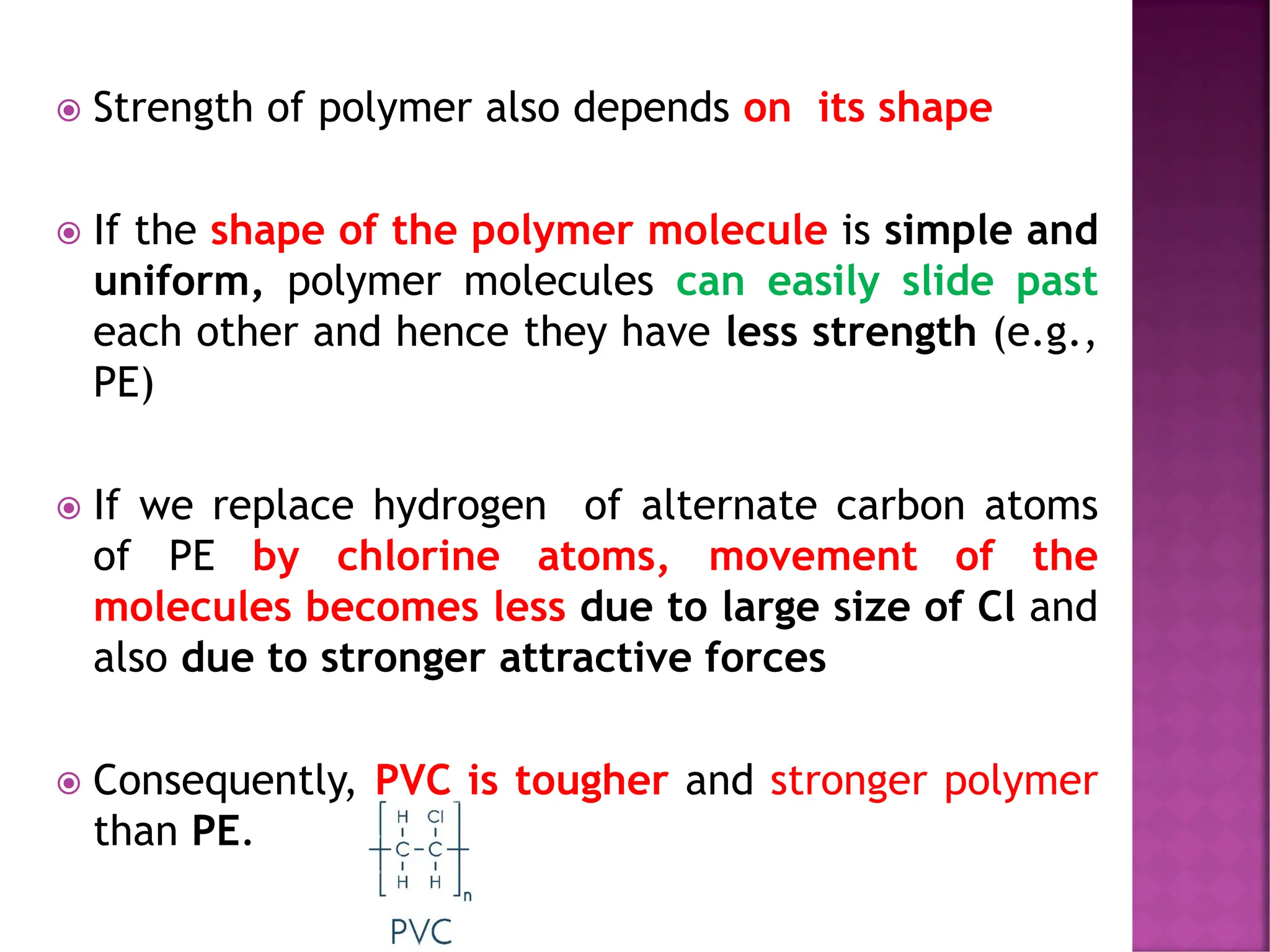
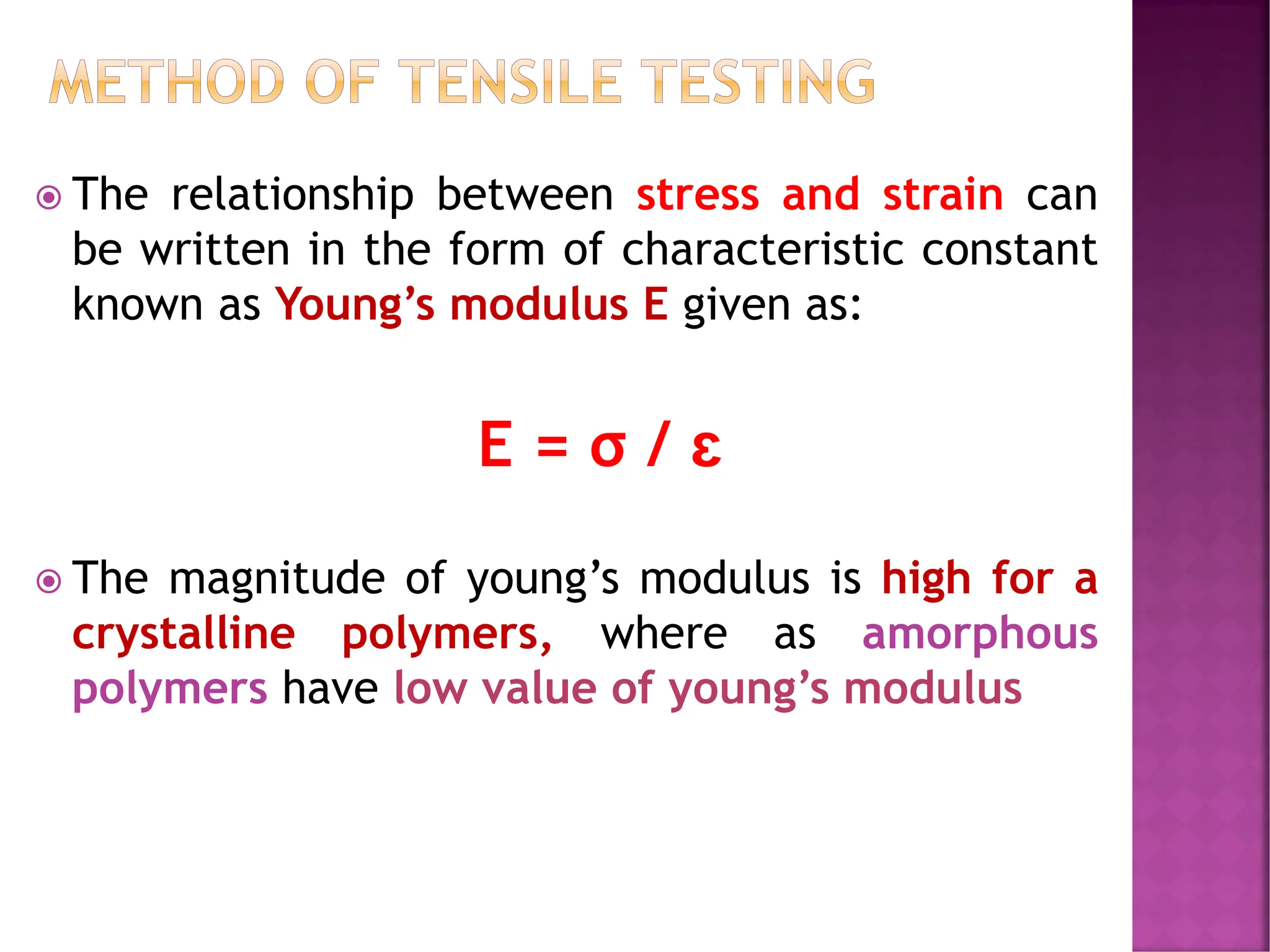
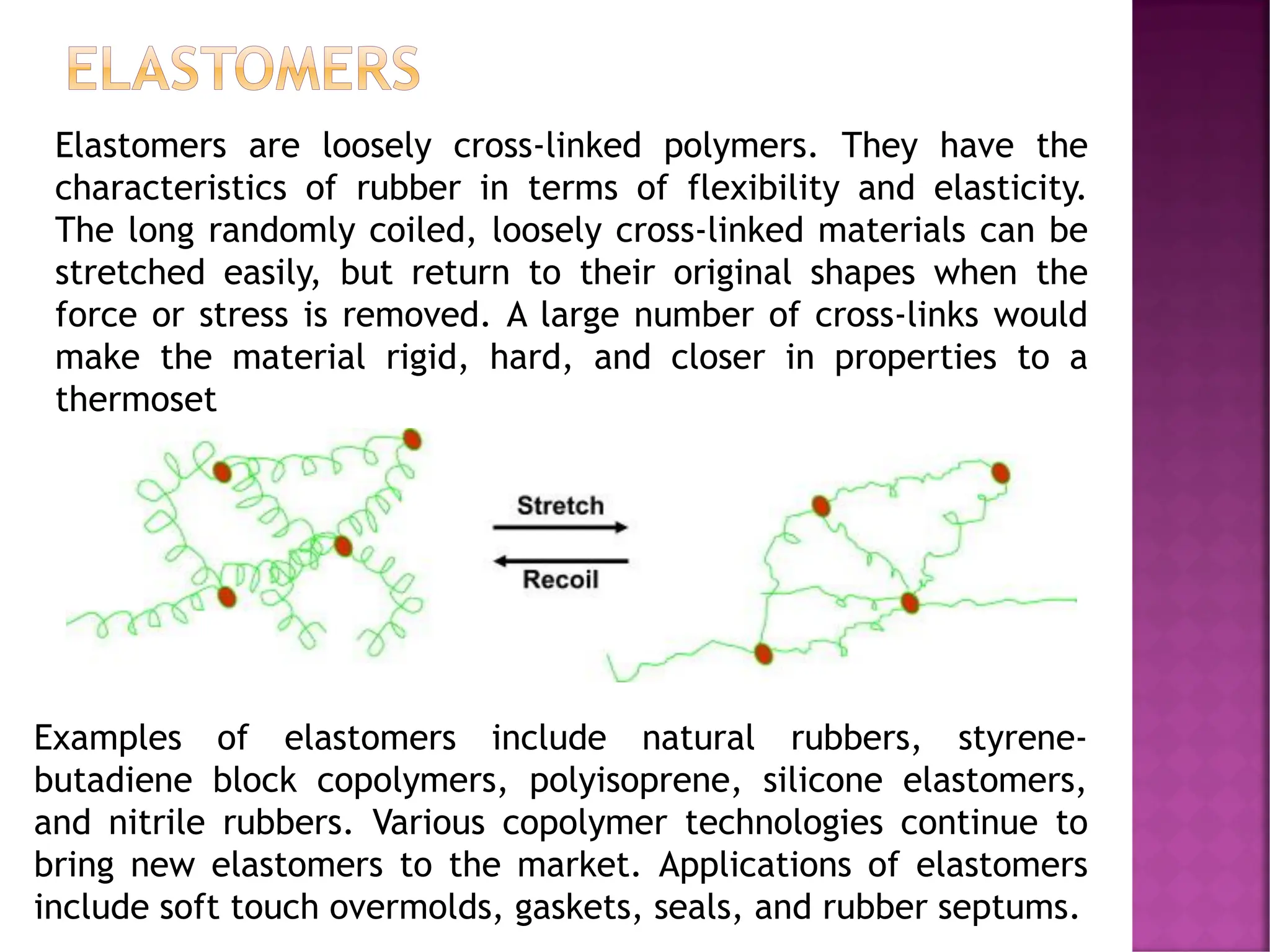
The document discusses the structure-activity relationship in polymers, highlighting how chemical structure affects various properties including solubility, reactivity, diffusion, electrical, optical, and mechanical characteristics. It emphasizes that polymers can act as insulators due to their bonding structure and provides insights into how environmental factors influence their properties. Additionally, the document details the mechanical testing of polymers, including stress-strain behavior and the characteristics of elastomers.