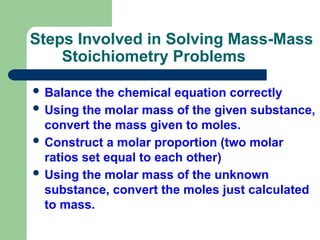
Stoichiometry is the quantitative study of reactants and products in chemical reactions, focusing on determining how much product can be formed from given reactants. Key steps include balancing equations, converting mass to moles, and using mole ratios to find the unknown quantities. The document provides examples and practice problems related to mass-mass and mass-volume stoichiometry calculations.







![Mole-Mole Problems
Using the practice question 2) above:
Equation of reaction
2C4H10 + 13O2 8CO2 + 10H2O
Mole ratio
C4H10 CO2
1 : 4 [ bases]
1.2 : X [ problem]
By cross-multiplication, X = 4.8 mols of CO2 given off](https://image.slidesharecdn.com/stoichiometryubb-240928115313-83cb5360/85/Stoichiometryforclasss11_ubb-pptx_28-09-24-9-320.jpg)
![Mole-Mass Problems
Problem 1: 1.50 mol of
KClO3 decomposes. How
many grams of O2 will be
produced? [k = 39, Cl =
35.5, O = 16]
2 KClO3 2 KCl + 3 O2](https://image.slidesharecdn.com/stoichiometryubb-240928115313-83cb5360/85/Stoichiometryforclasss11_ubb-pptx_28-09-24-10-320.jpg)
![Three steps…Get Your
Correct Answer
Use mole ratio
Get the answer in moles and then
Convert to Mass. [Simple Arithmetic]
Hello!
If you are given a mass in the problem,
you will need to convert this to moles
first. Ok?](https://image.slidesharecdn.com/stoichiometryubb-240928115313-83cb5360/85/Stoichiometryforclasss11_ubb-pptx_28-09-24-11-320.jpg)








![Practice Problems
As per the equation,
Mole ratio 1 : 1
problem 0.03125mol X
X = 0.03125mol of CO2
Convert mole to volume [slide 17]
Volume = (0.03125 x 22.4)dm3](https://image.slidesharecdn.com/stoichiometryubb-240928115313-83cb5360/85/Stoichiometryforclasss11_ubb-pptx_28-09-24-20-320.jpg)