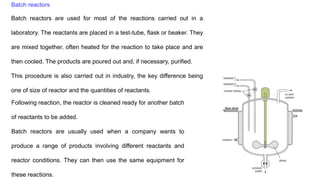
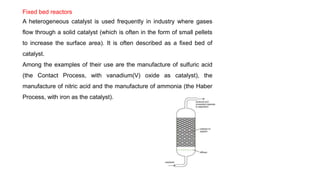
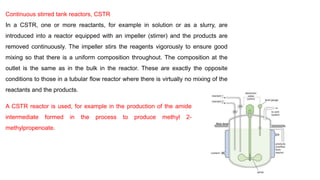
The document discusses various types of chemical reactors used in industry, including batch and continuous reactors, and their design considerations based on thermodynamics and kinetics. Batch reactors are mainly used in laboratories for small-scale reactions, while continuous reactors allow for more efficient operations with consistent product quality and lower waste. Different continuous reactor types such as tubular, fixed bed, fluid bed, and continuous stirred tank reactors are described, highlighting their specific applications and advantages.