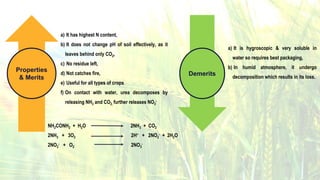
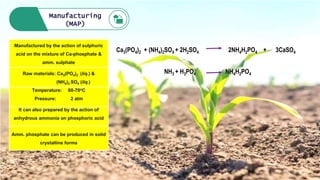
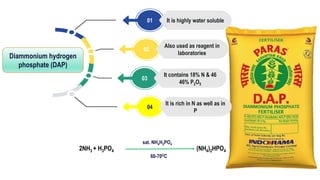
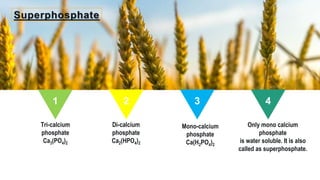
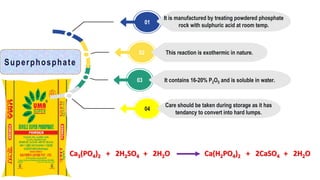
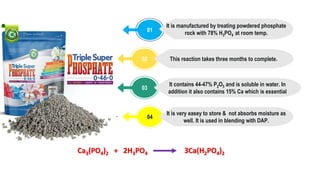
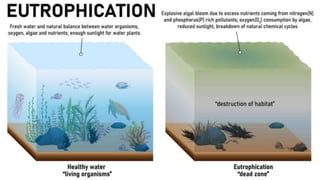
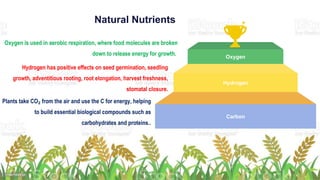
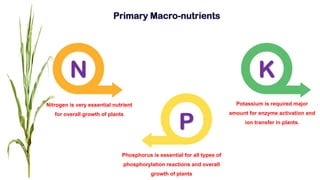
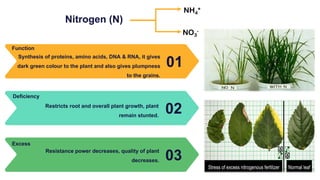
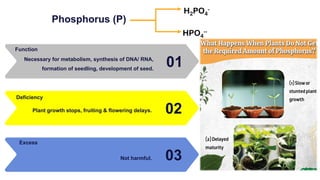
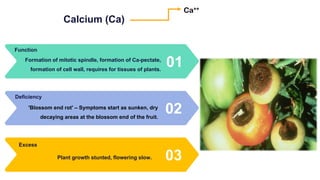
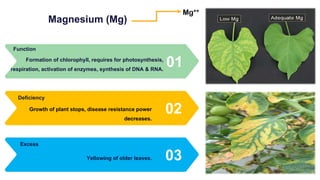
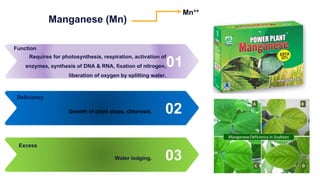
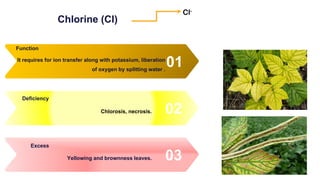
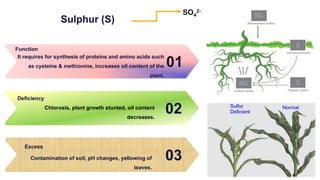
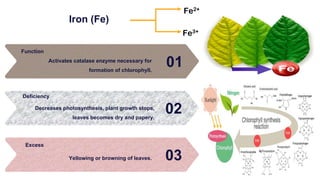
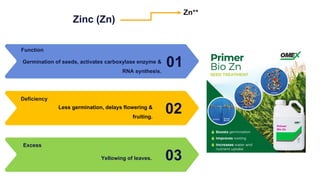
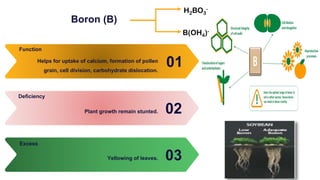
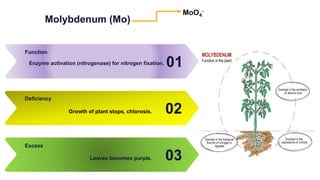
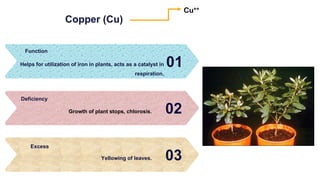
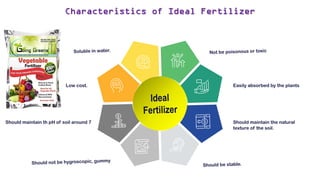
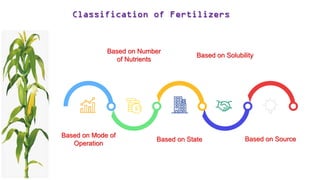
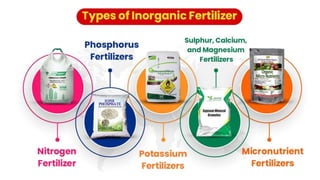
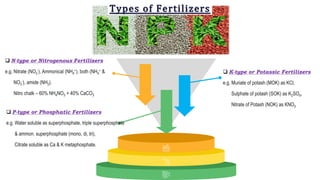
Fertilizers are chemical compounds that enhance soil fertility by providing essential nutrients like nitrogen, phosphorus, and potassium, which promote plant growth and productivity. The document outlines the historical development of fertilizers and their classification, including nitrogenous, phosphatic, and potassic types, along with their manufacturing processes and advantages. Additionally, it addresses the environmental impacts of fertilizers, such as soil and water pollution due to excess nutrient runoff.






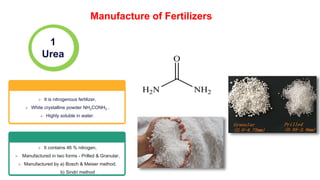
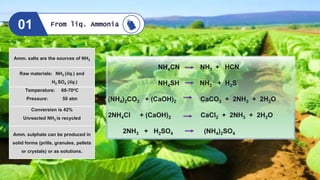
![Raw materials: NH3 (liq.) and
CO2 (g)
Temperature : 180-185oC
Pressure: 180-200 atm
Ist step: Carbamate formation
IInd step: Urea conversion
Urea can be produced in solid forms
(prills, granules, pellets or crystals)
or as solutions.
Developed by Carl Bosch & W.
Meiser in 1922
Urea plant using ammonium carbamate, Fixed Nitrogen
Research Laboratory (FNRC), California 1930.
2 NH3 + CO2 ⇌ [NH4]+[NH2COO]− (ΔH = −117 kJ/mol at 180 atm and 180 °C)
[NH4]+[NH2COO]− ⇌ CO(NH2)2 + H2O (ΔH = +17.5 kJ/mol at 180–185 °C)
Bosch - Meiser method
01](https://image.slidesharecdn.com/fertilizers-240925110803-362829f9/85/B-Sc-_Fertilizers_ubb_25-09-24_____________________-28-320.jpg)
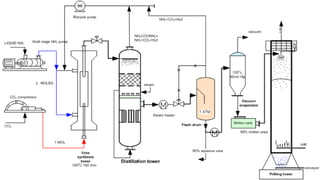


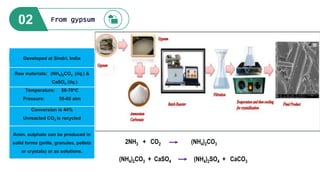
![Raw materials: NH3 (liq.) and
CO2 (liq.)
Temperature: 180-182oC
Pressure: 170-200 atm
Conversion is 37%
Unreacted CO2 is recycled
Urea can be produced in solid forms
(prills, granules, pellets or crystals)
or as solutions.
Developed at Sindri, India
2 NH3 + CO2 + H2O (NH4) 2CO3
(NH4) 2CO3 + 2 HNO3 2NH4NO3 + CO2 + H2O
Sindri method
02
2 NH3 + CO2 ⇌ [NH4]+[NH2COO]− (ΔH = −117 kJ/mol at 180 atm and 180 °C)
[NH4]+[NH2COO]− ⇌ CO(NH2)2 + H2O (ΔH = +15.5 kJ/mol at 180–185 °C)](https://image.slidesharecdn.com/fertilizers-240925110803-362829f9/85/B-Sc-_Fertilizers_ubb_25-09-24_____________________-32-320.jpg)