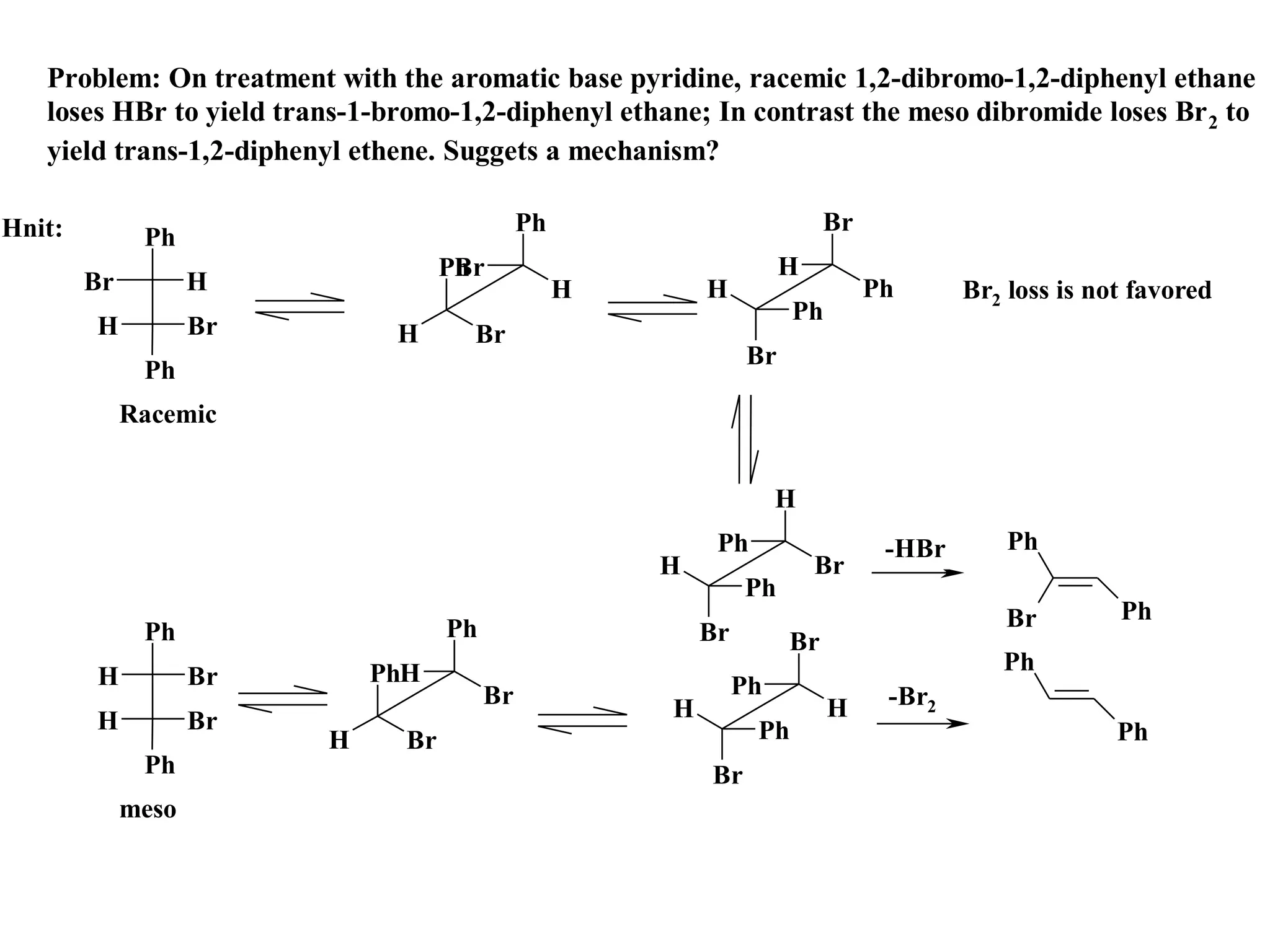
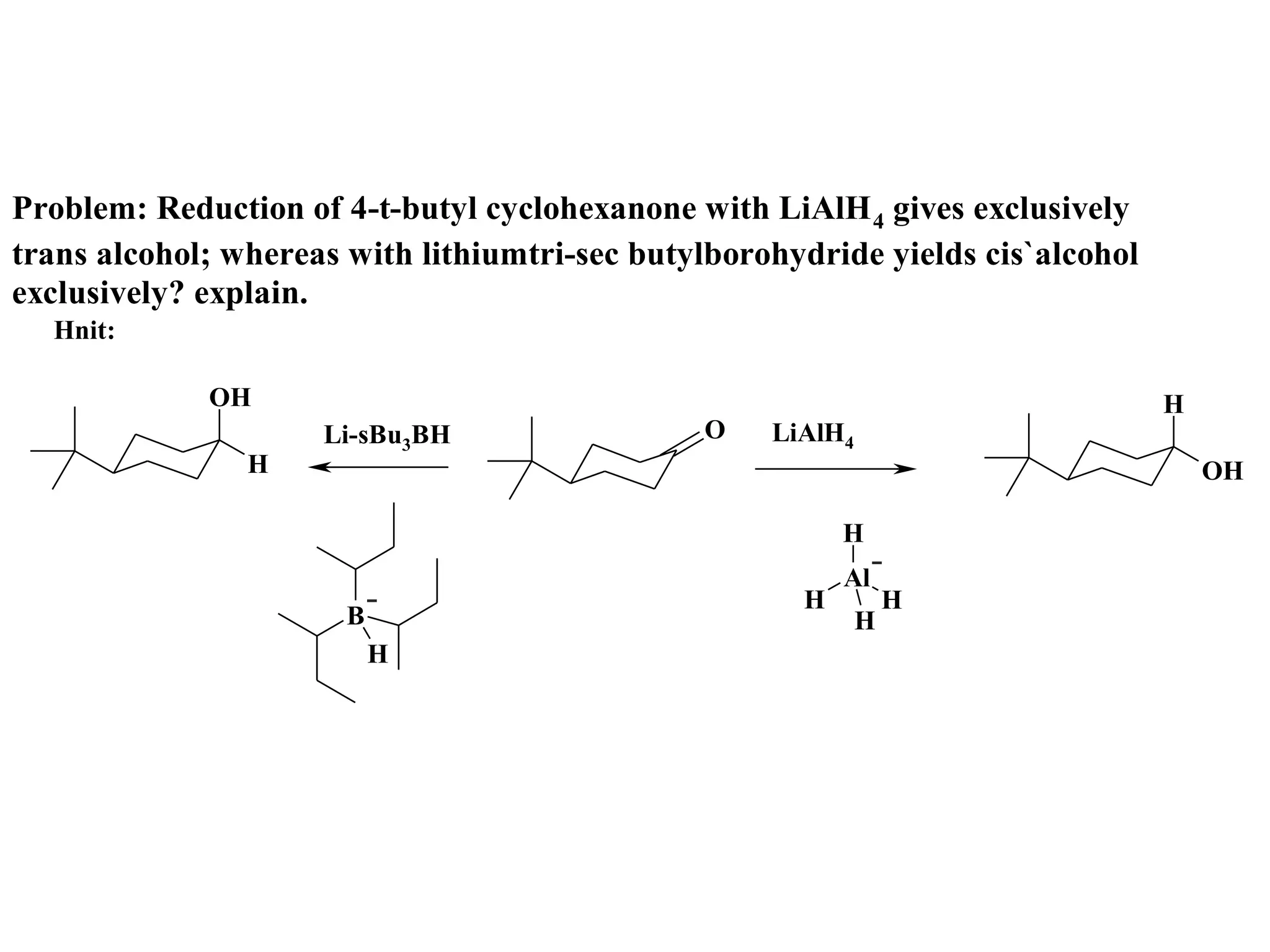
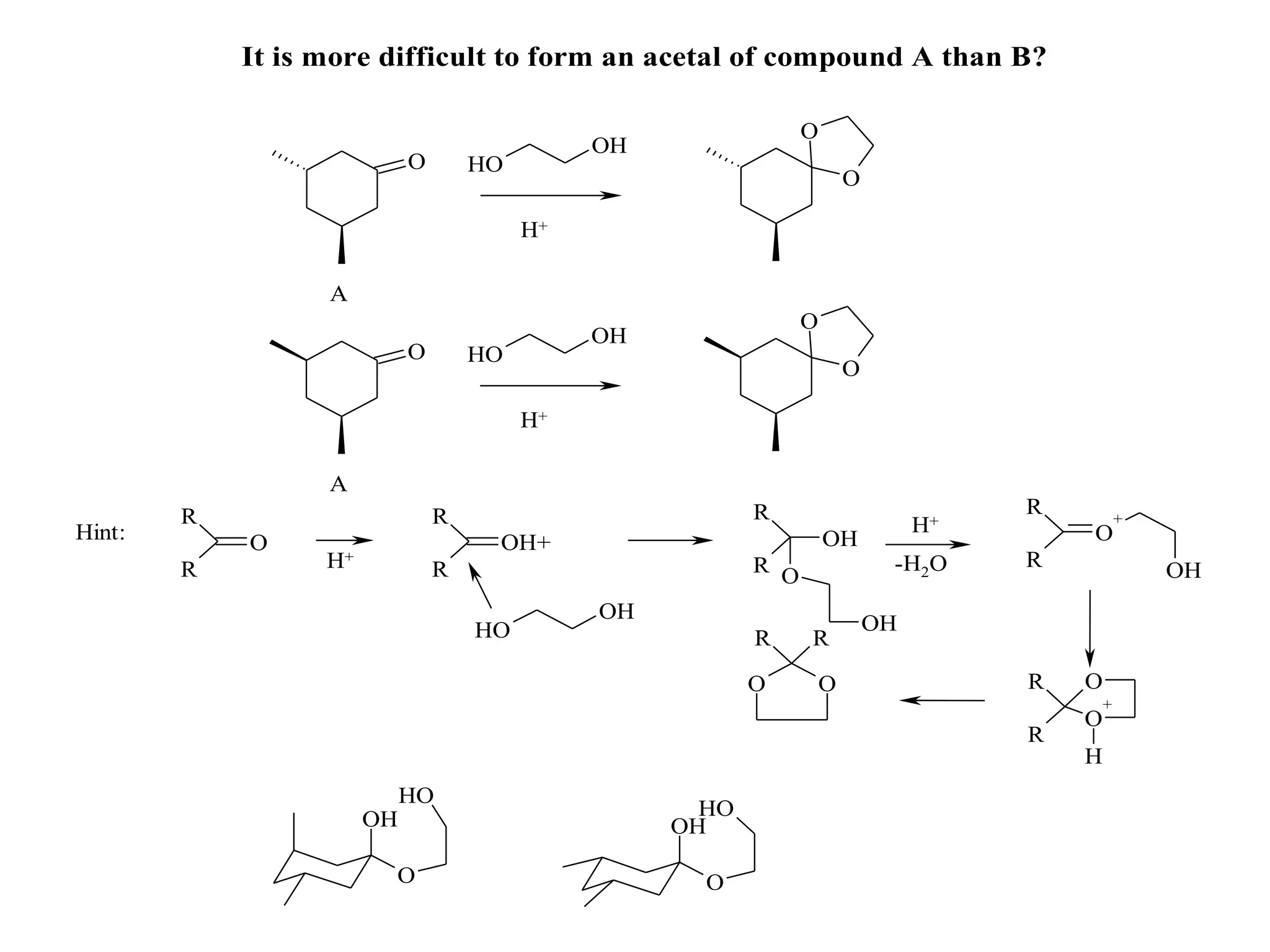
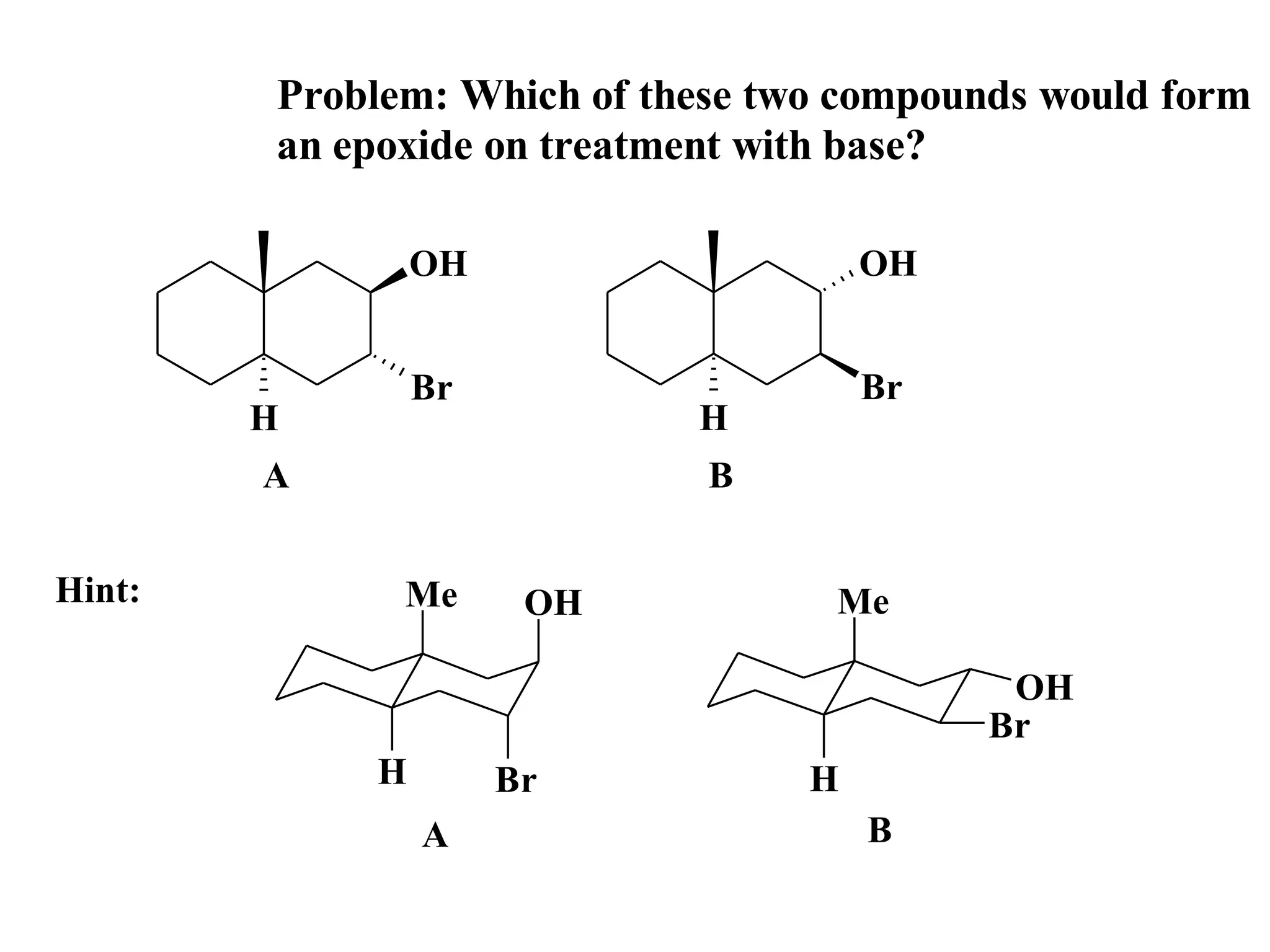
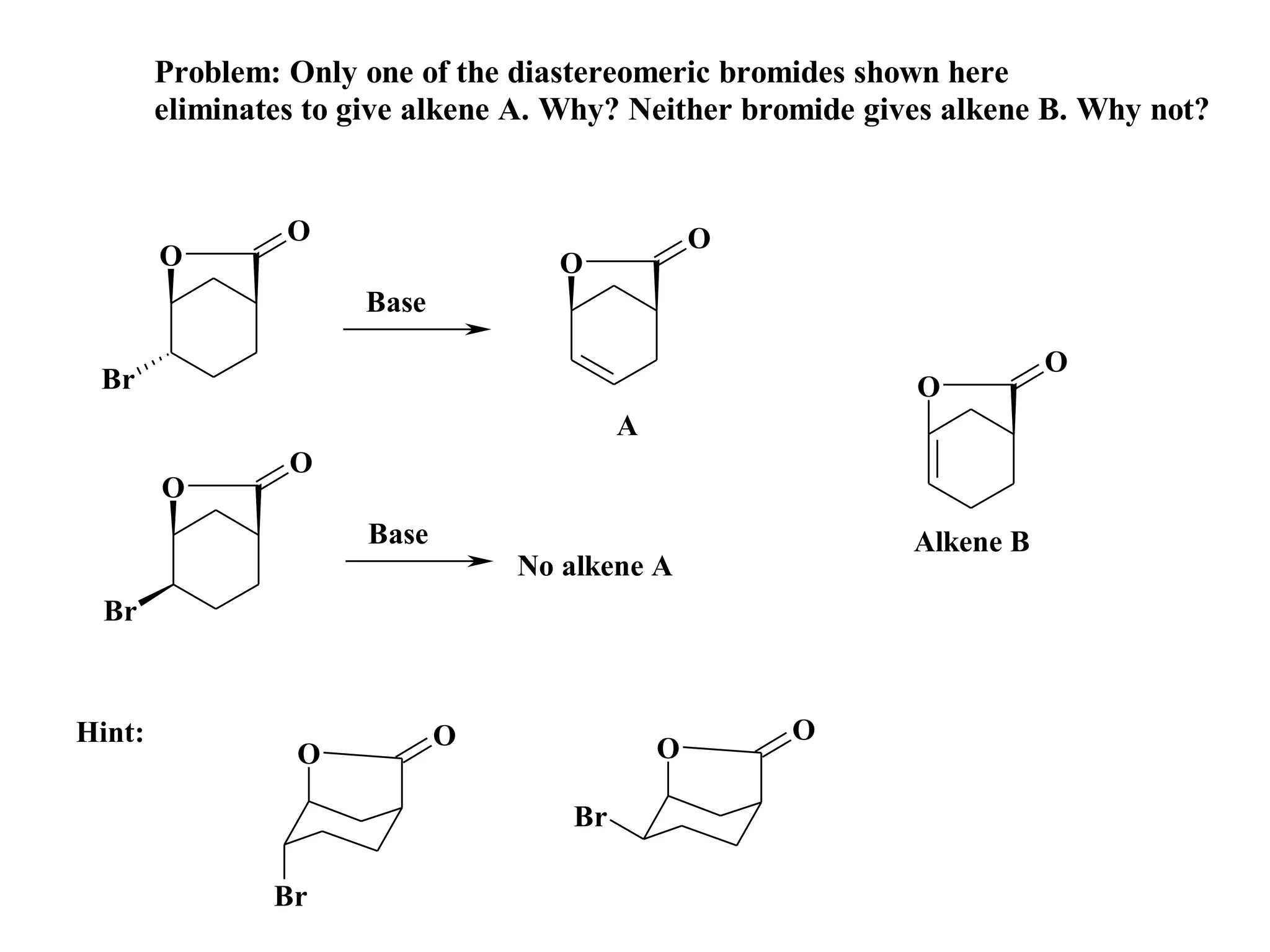
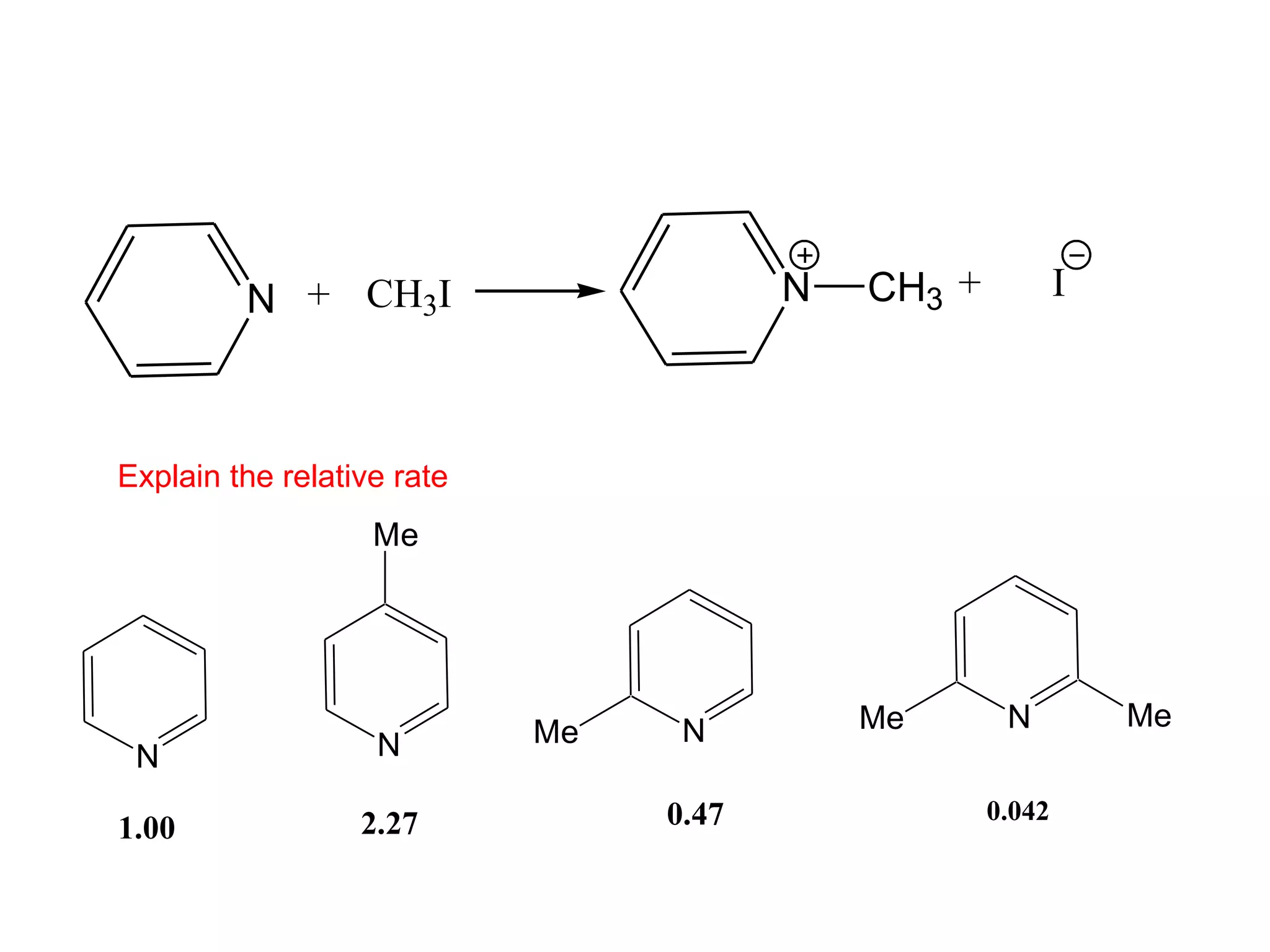
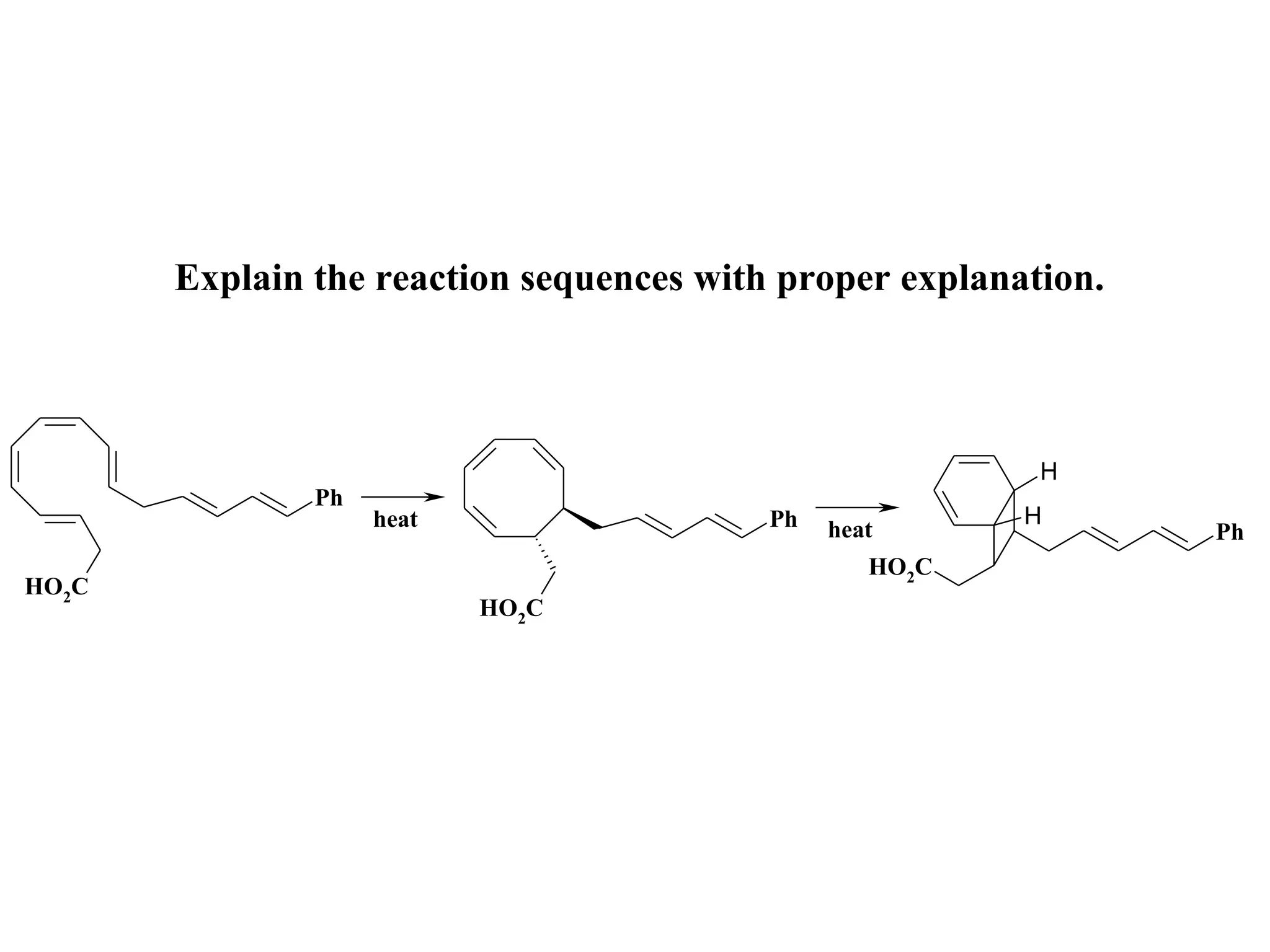
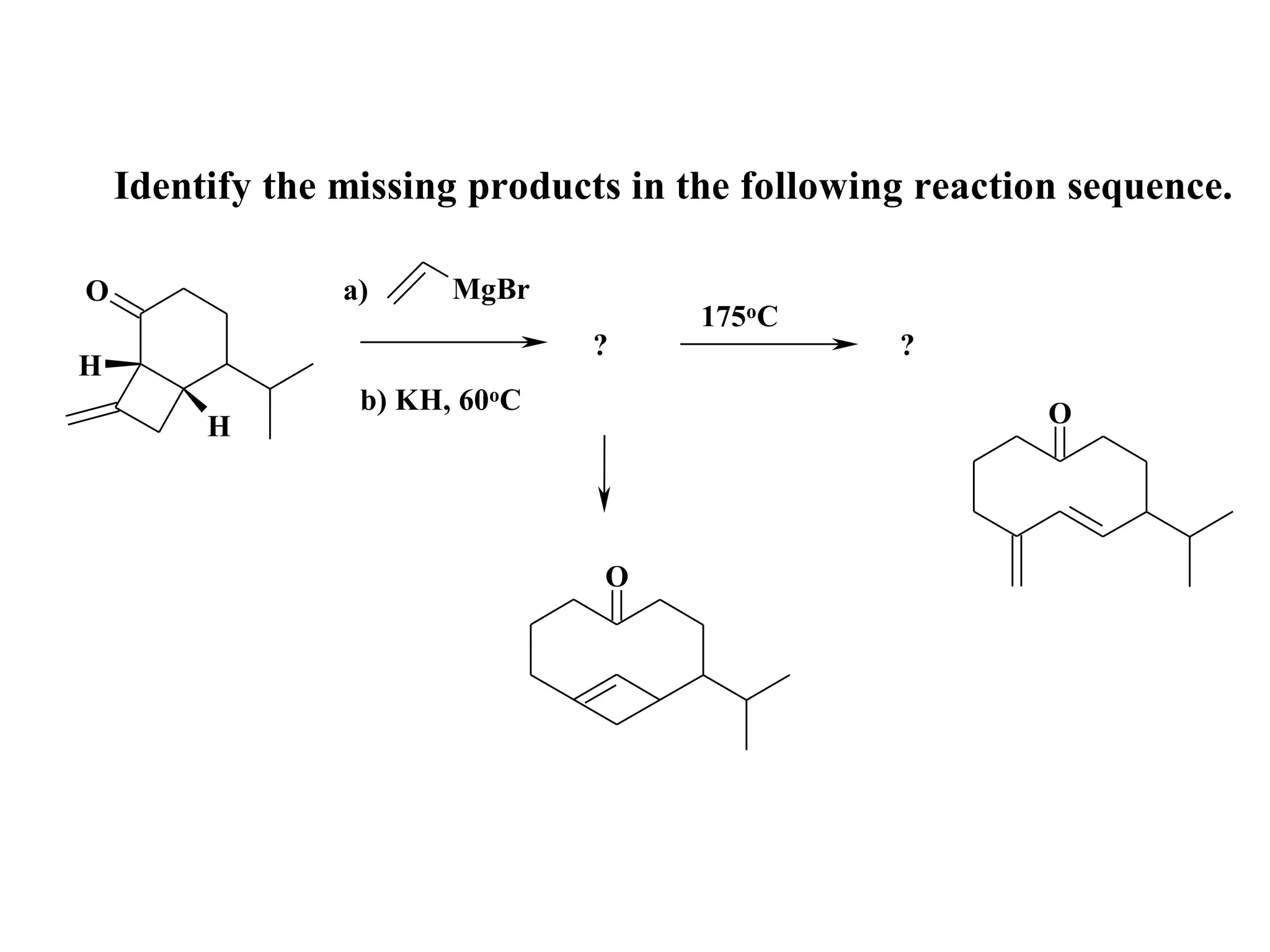
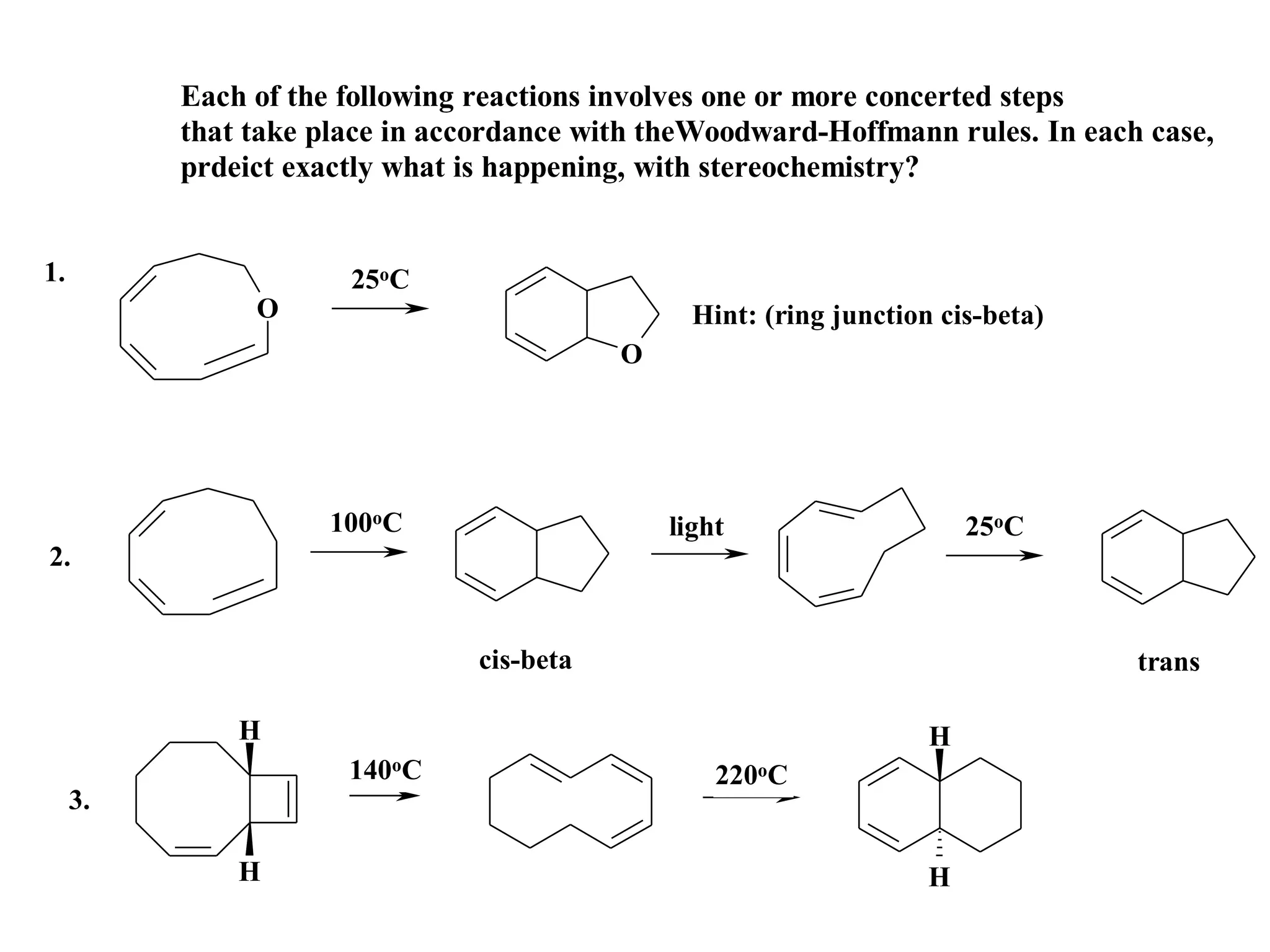
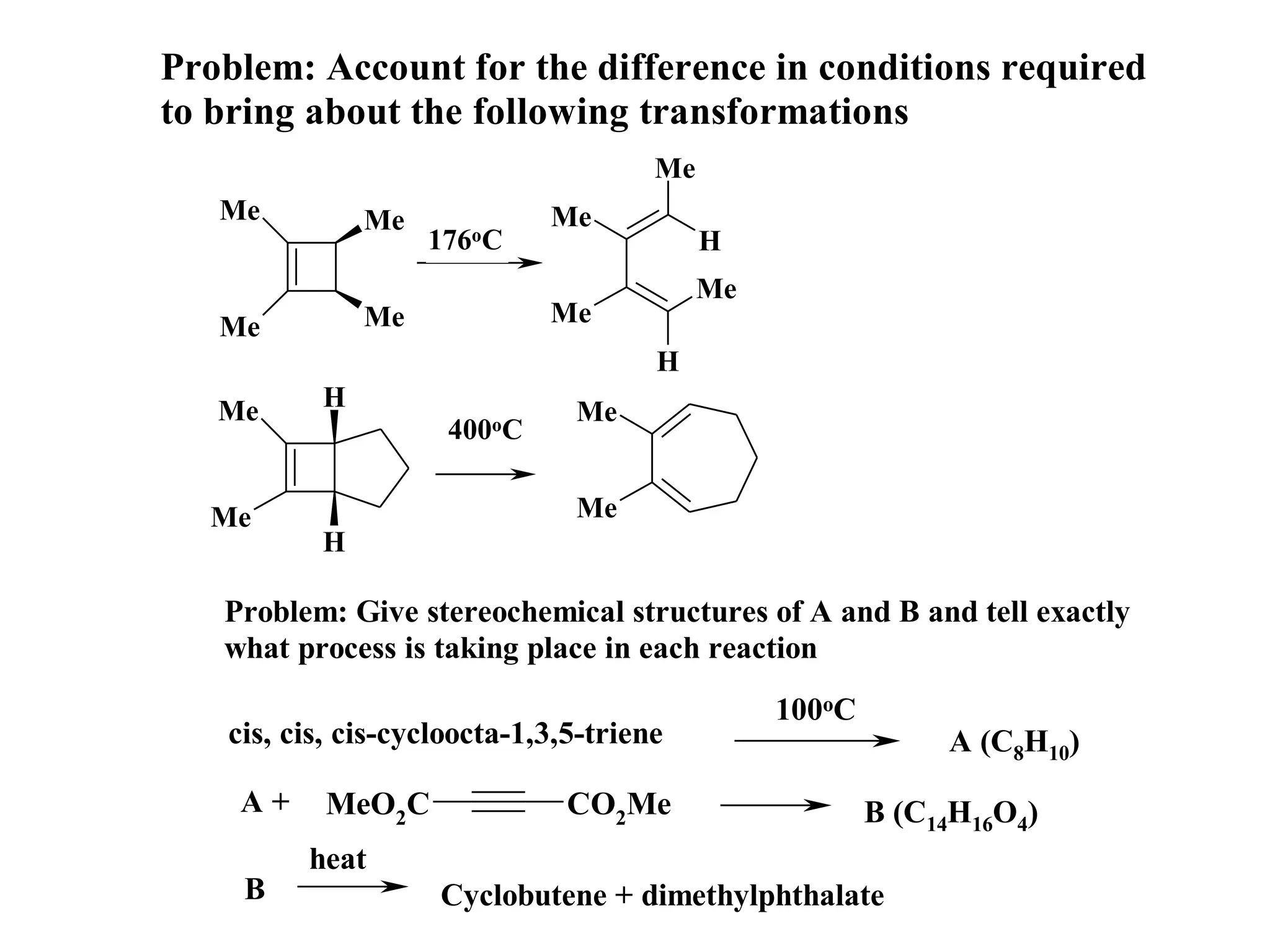
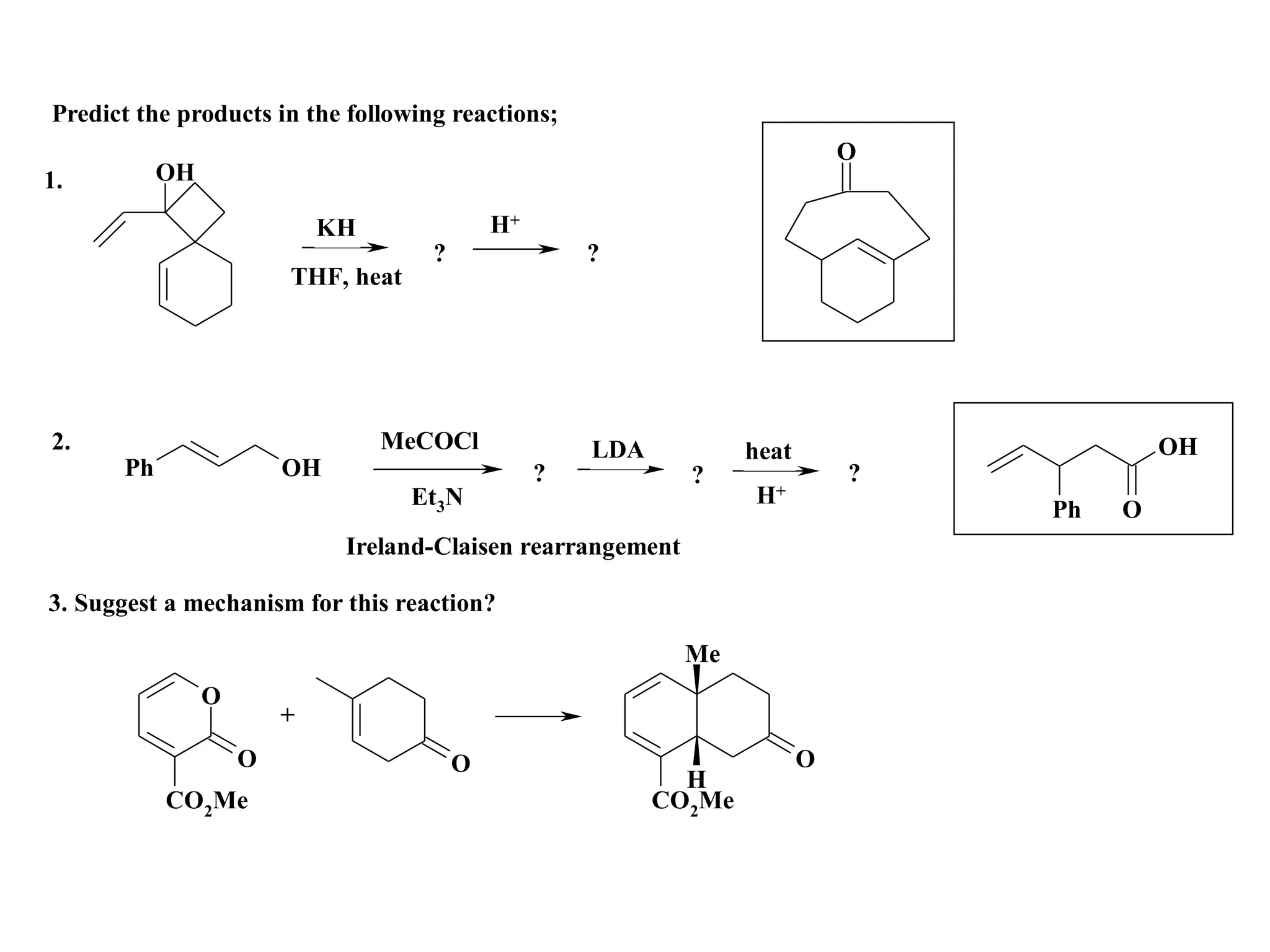
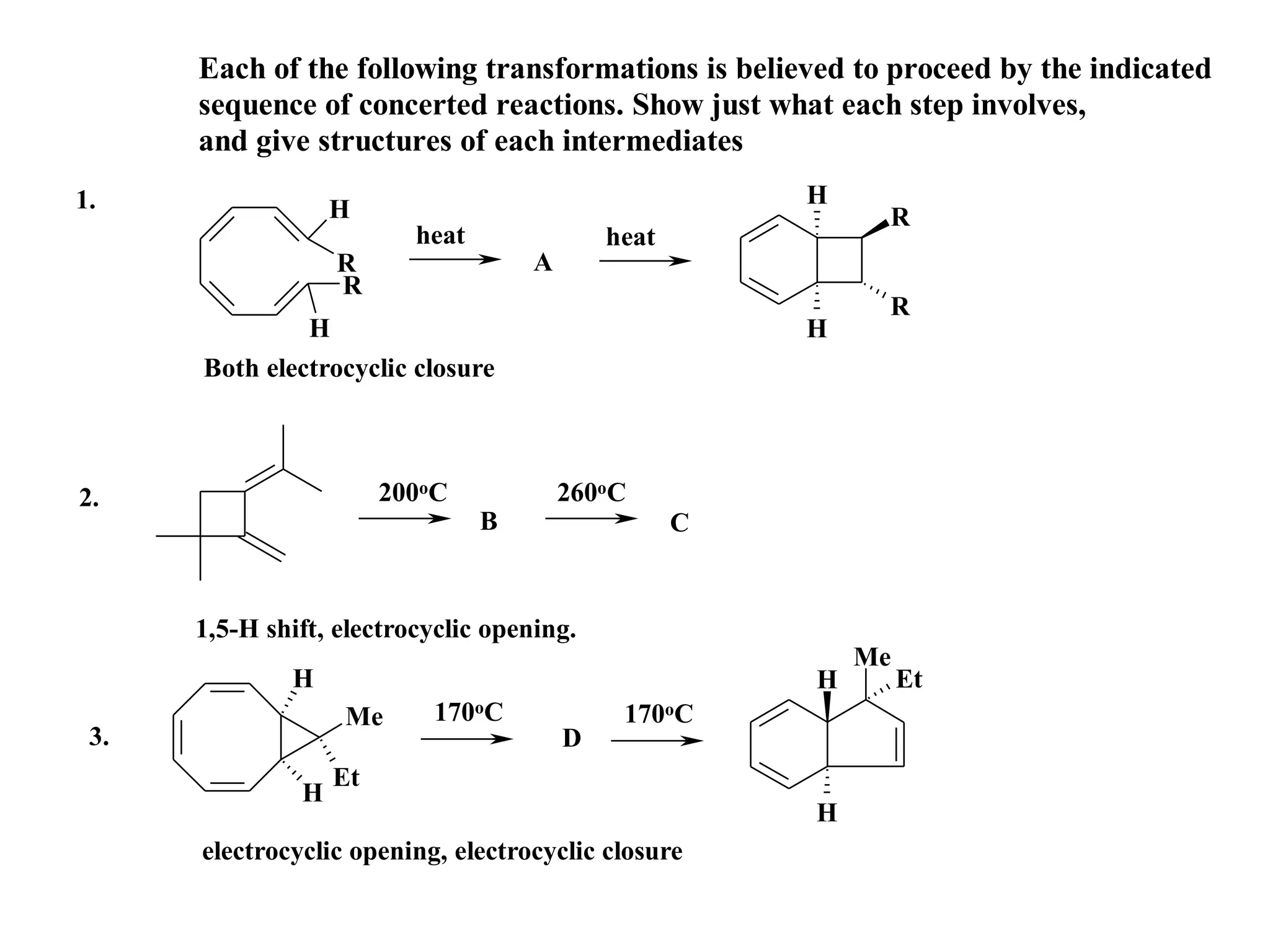
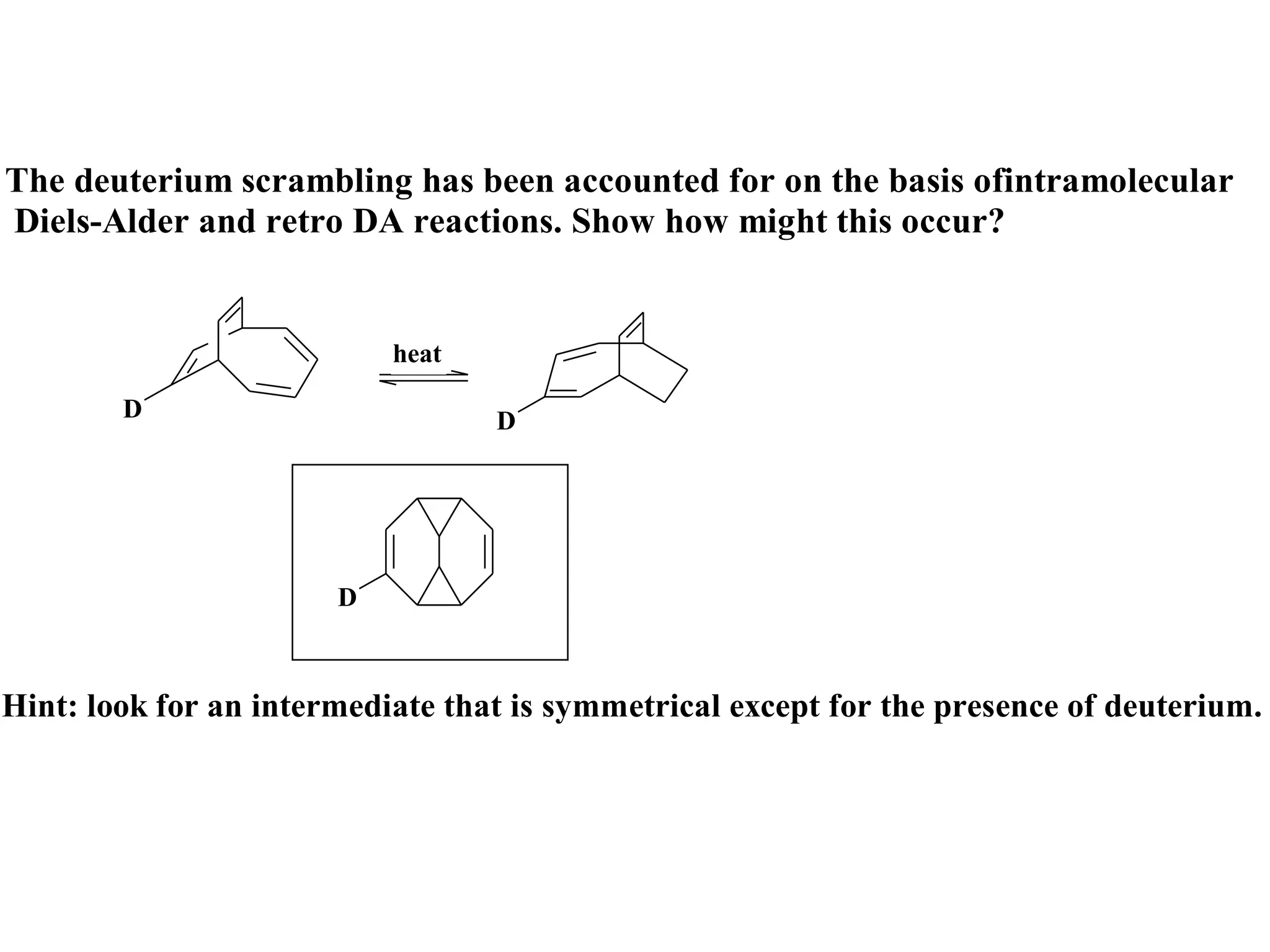
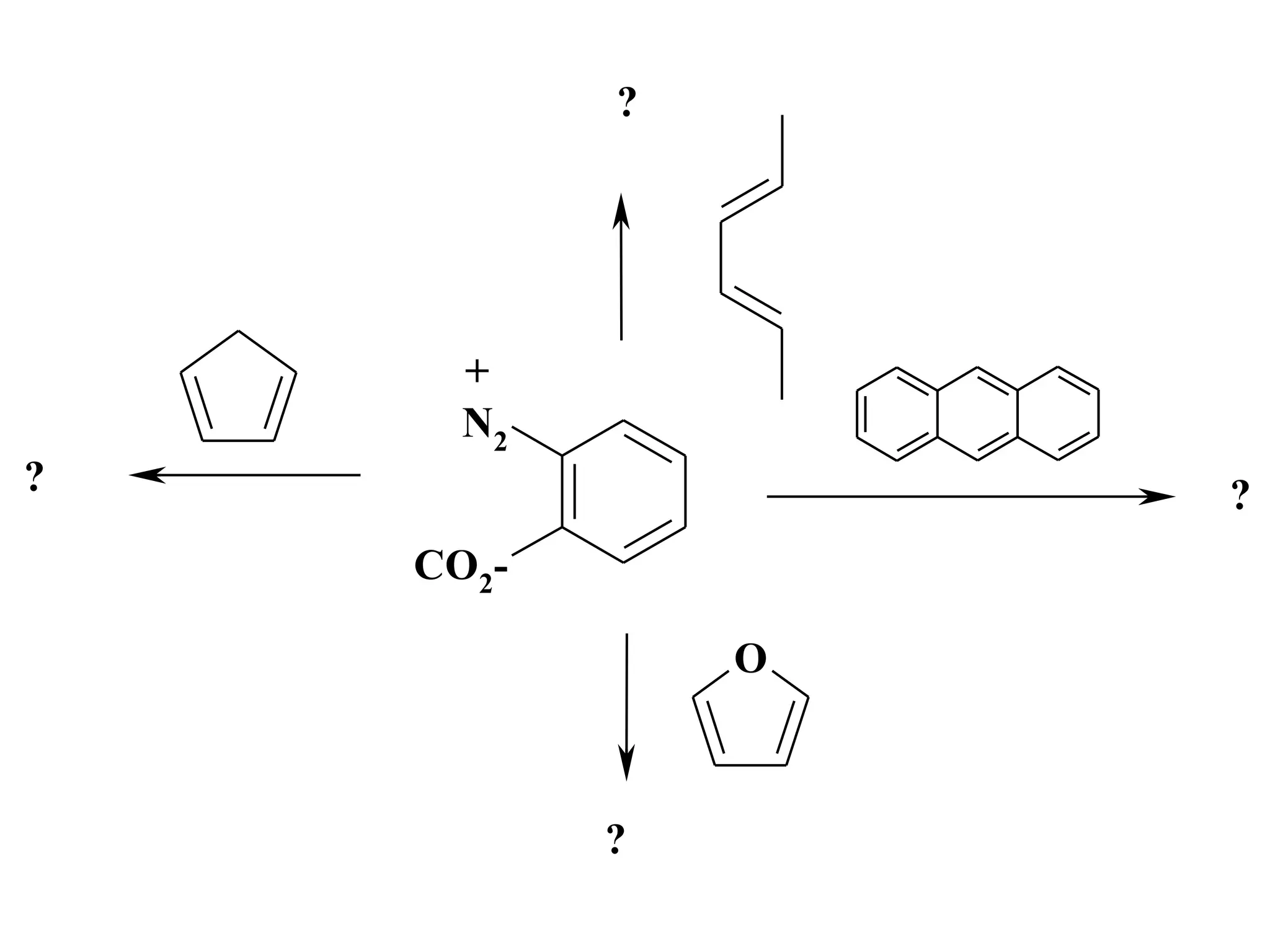
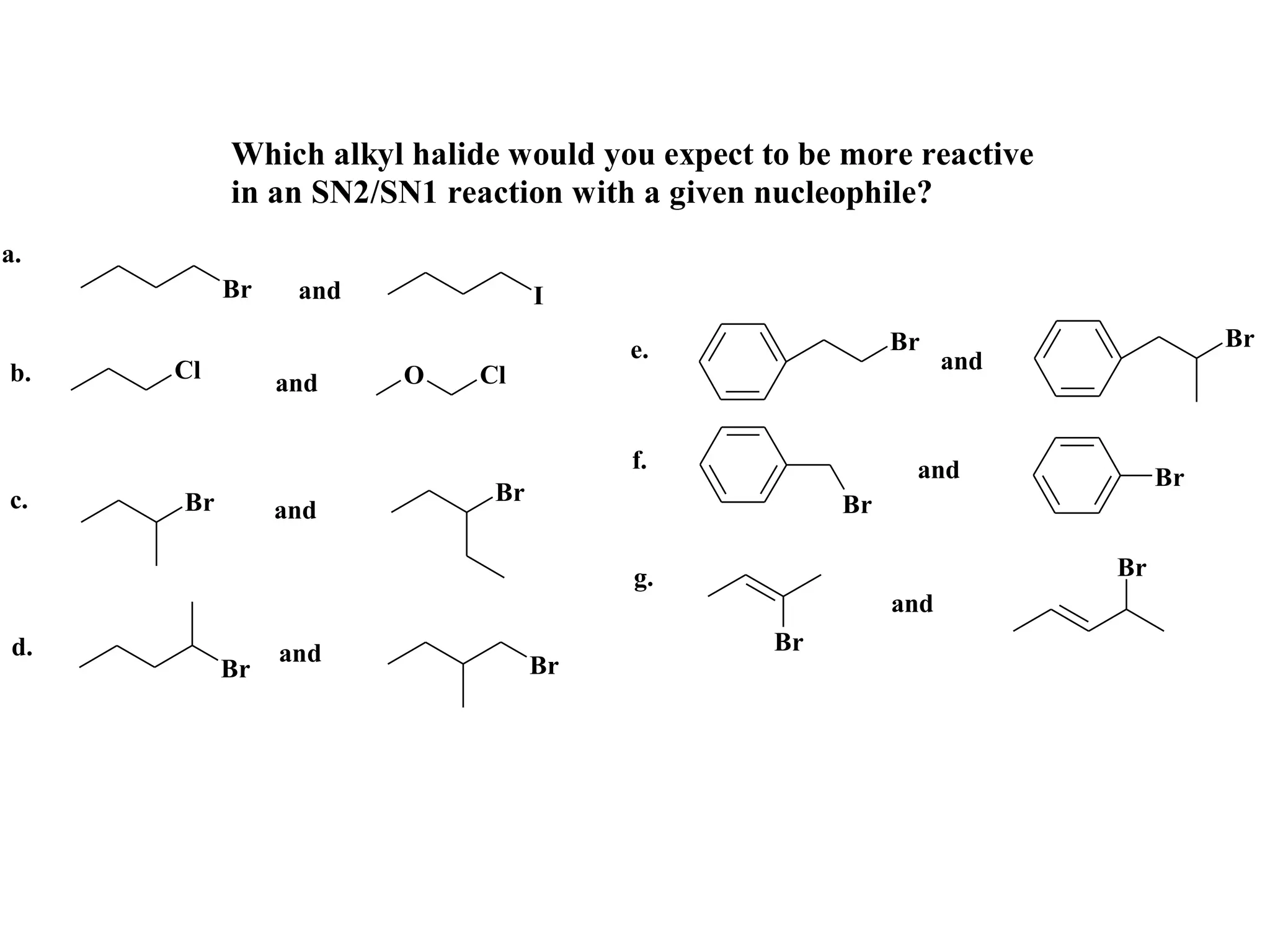
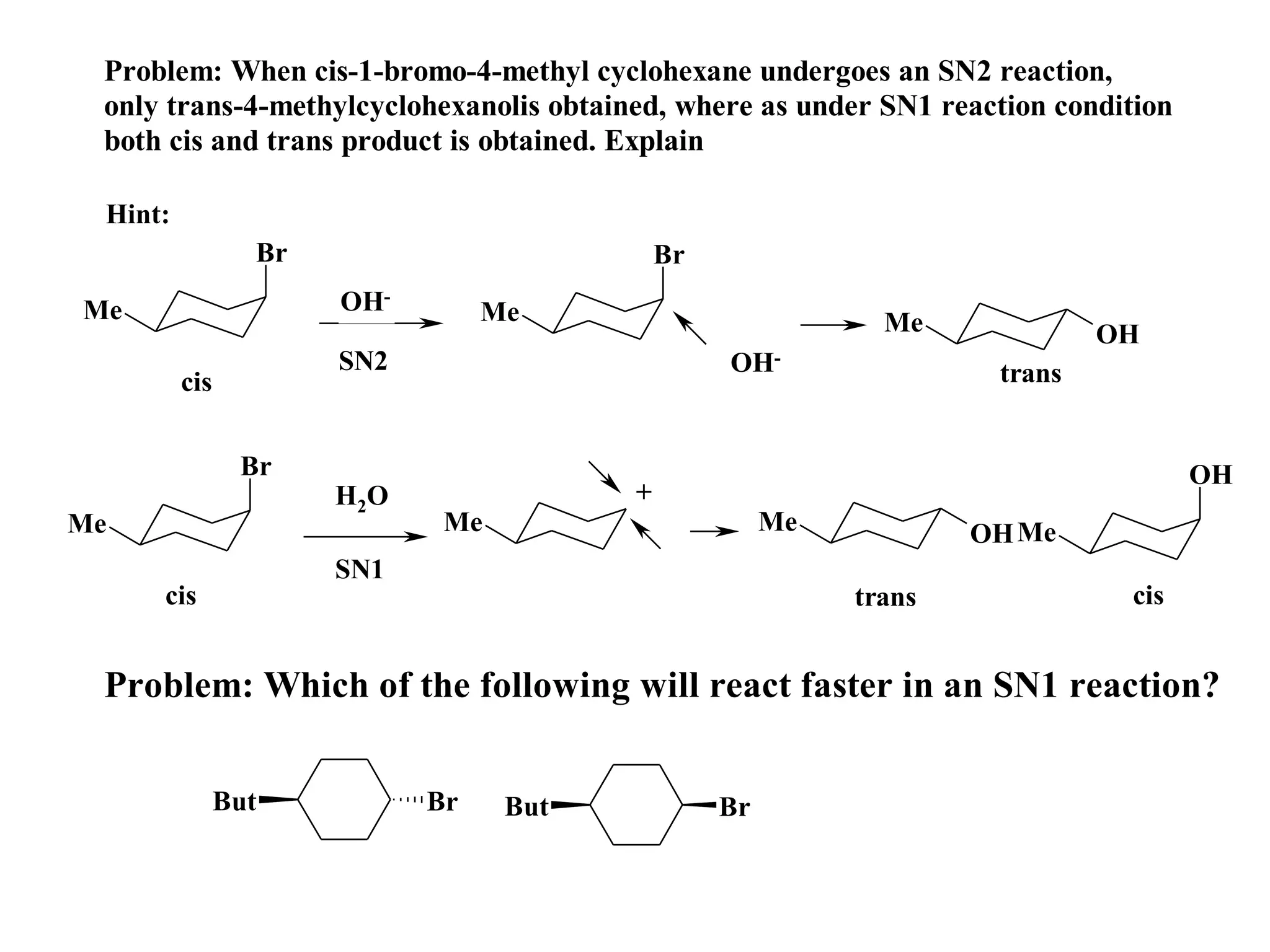
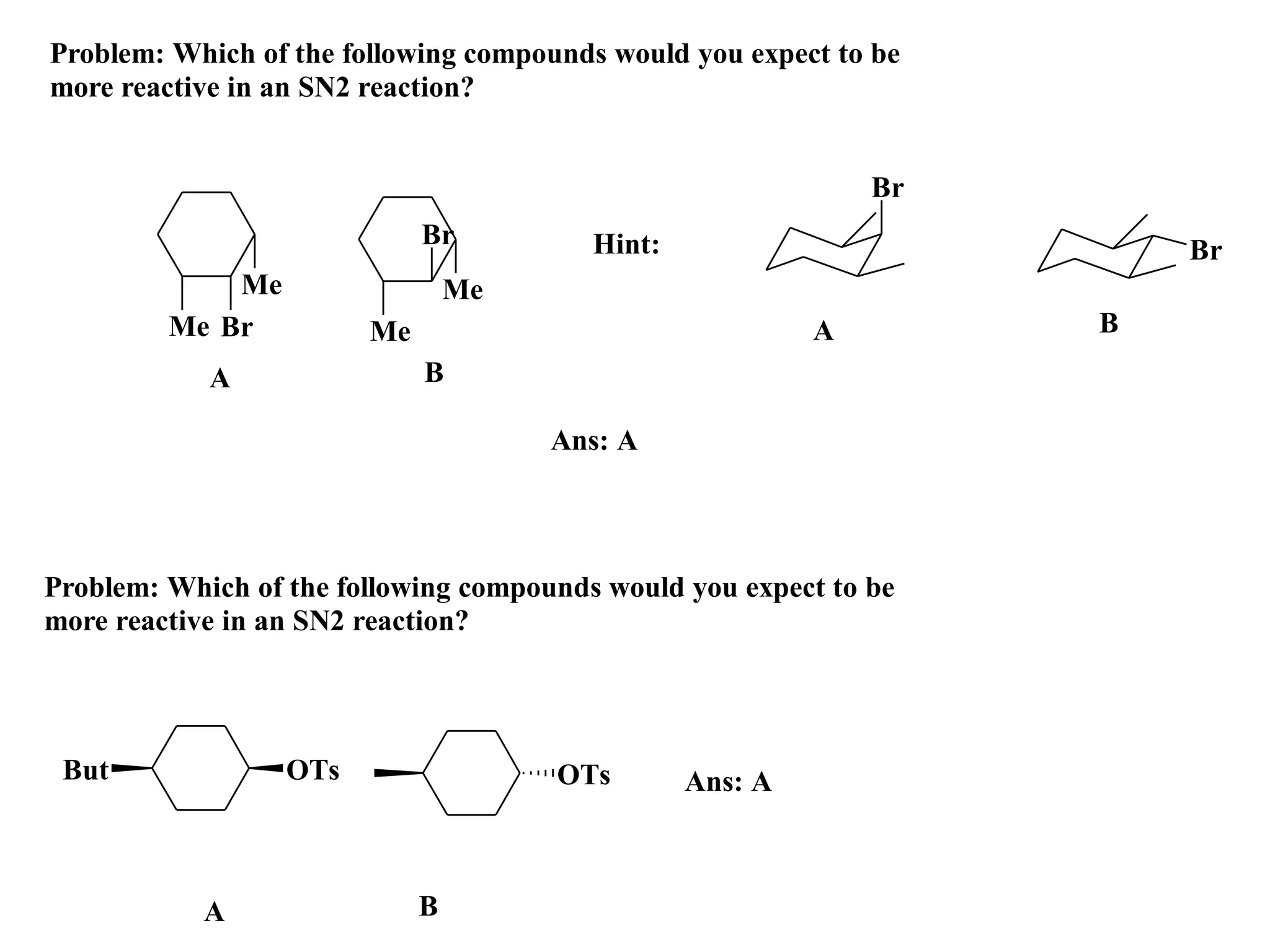
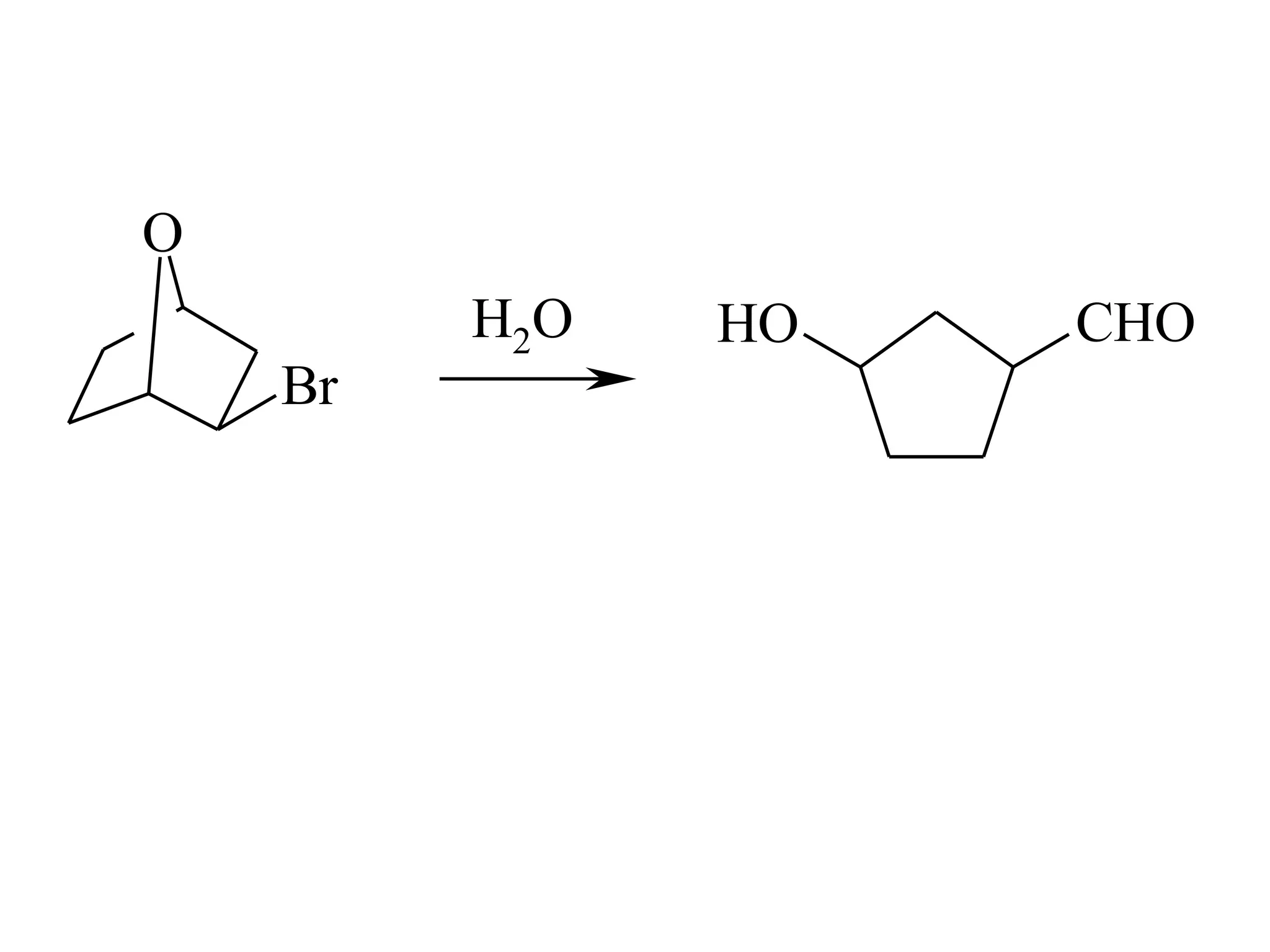
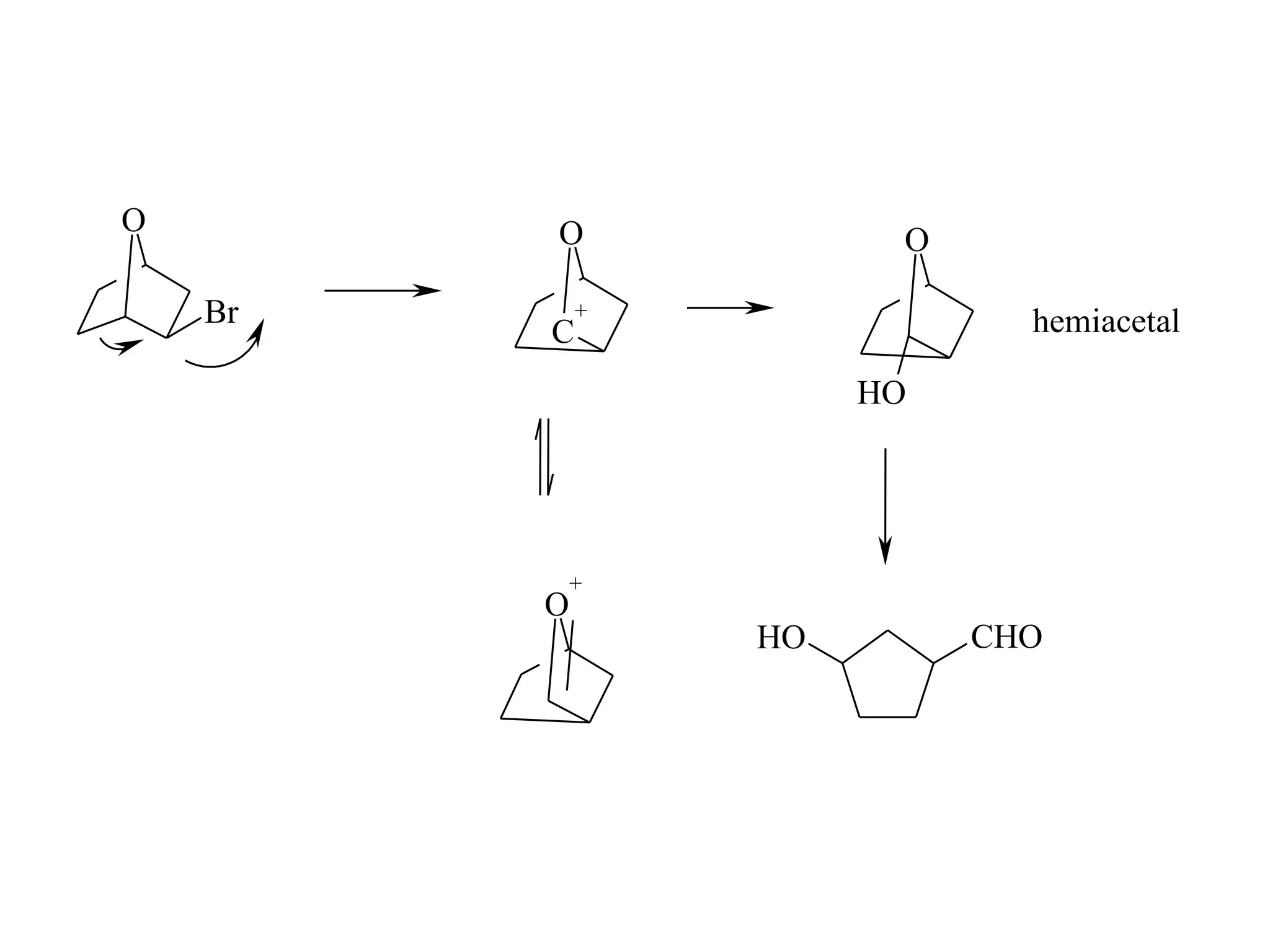
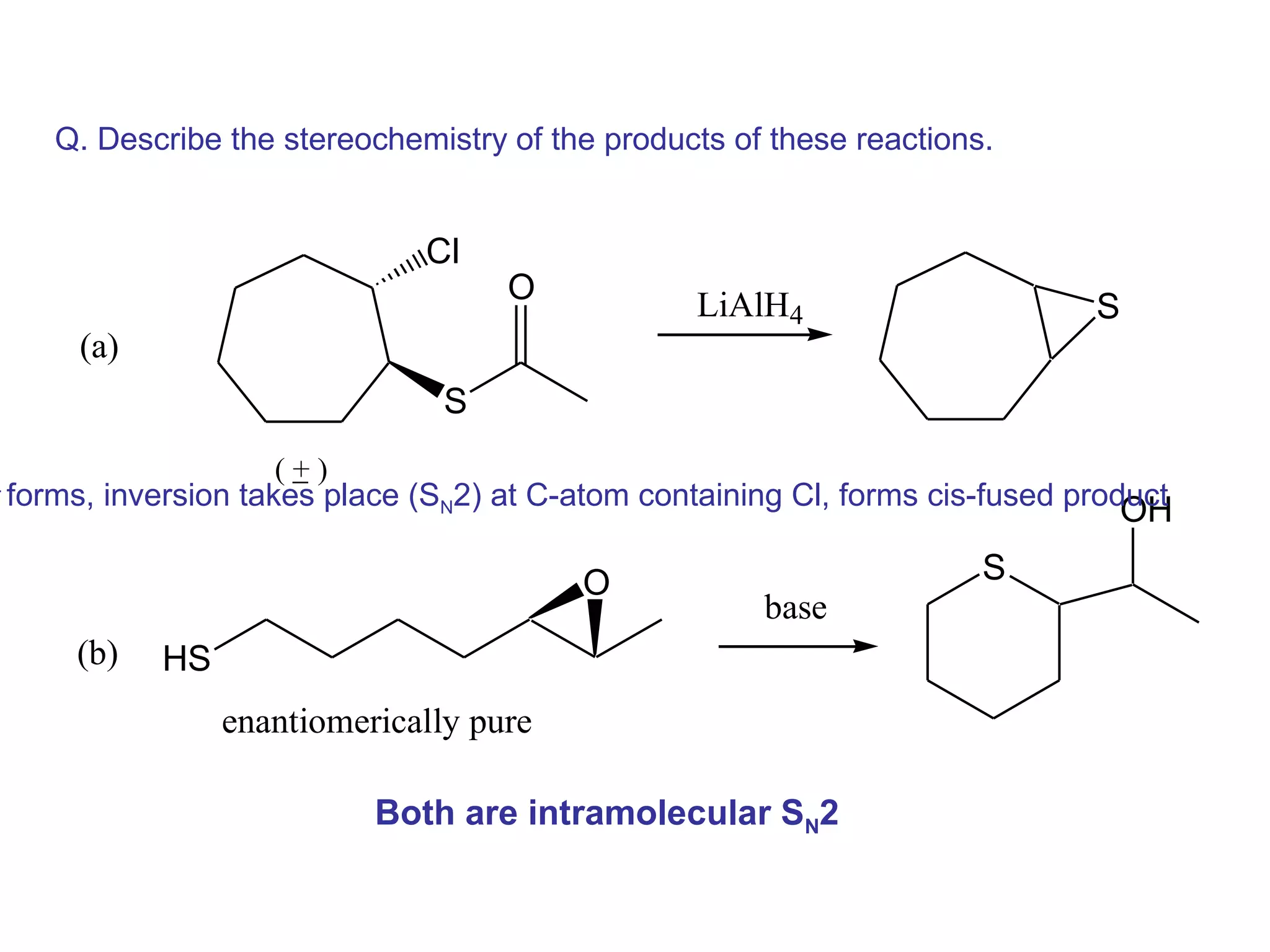
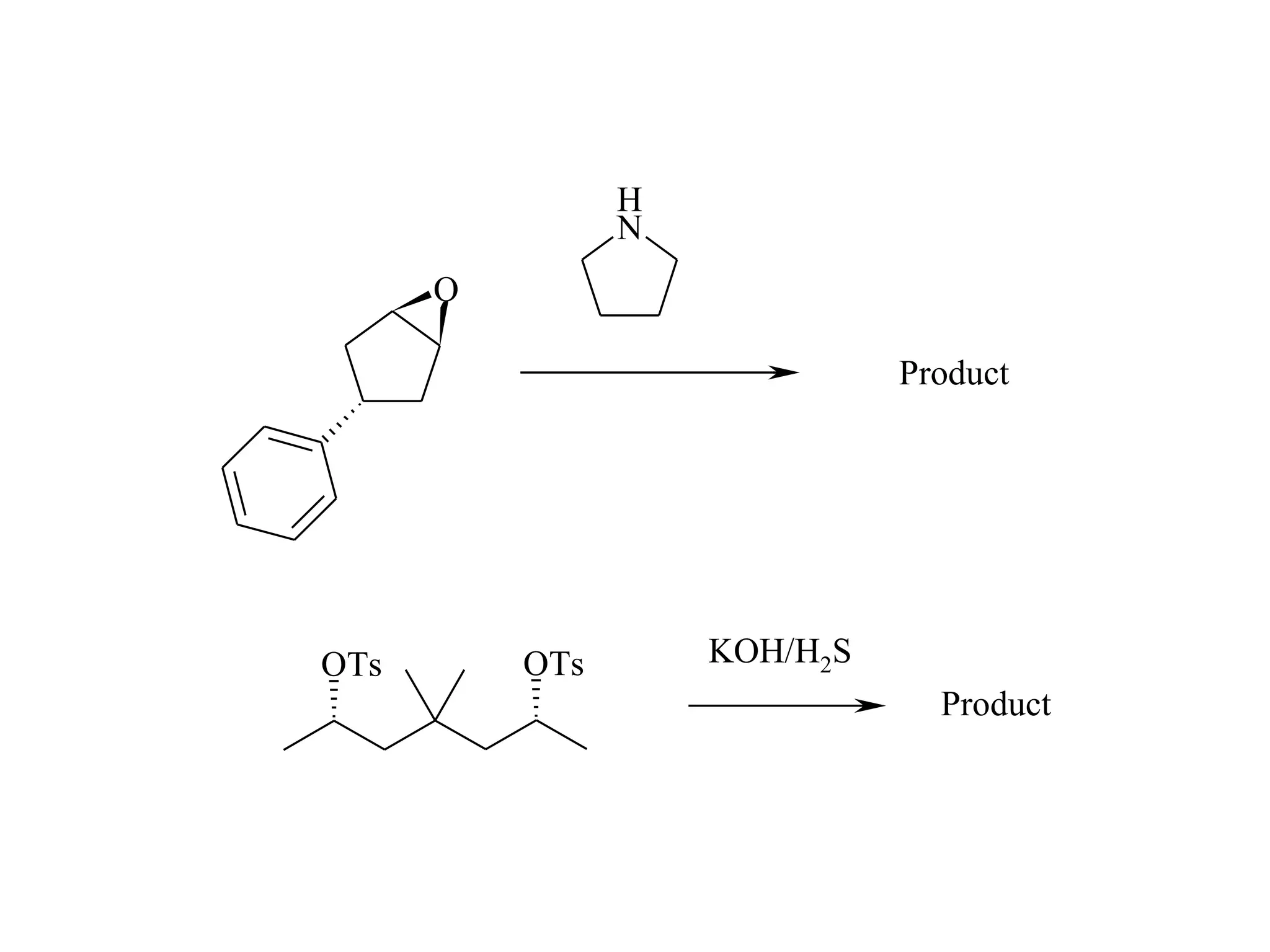
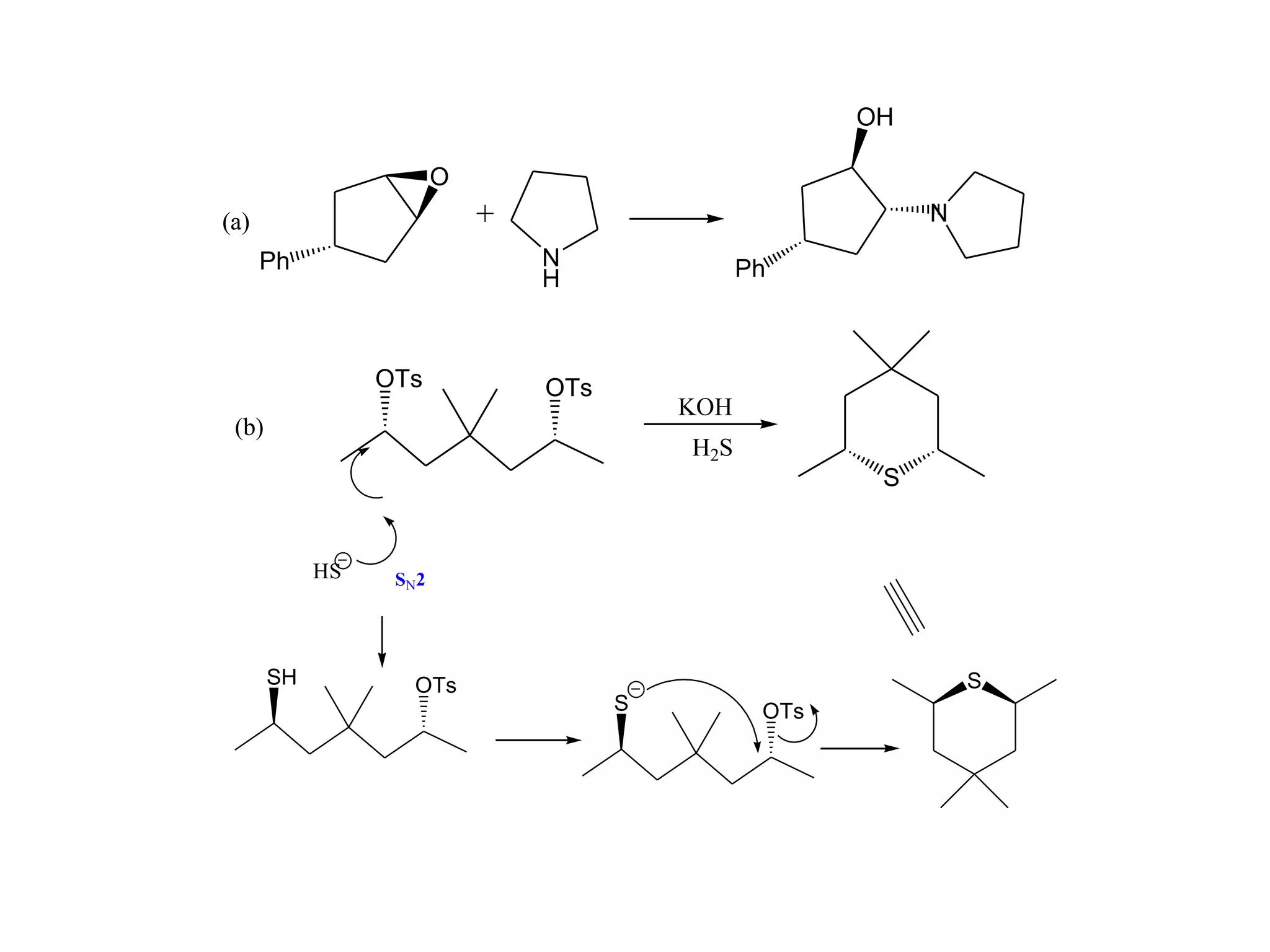
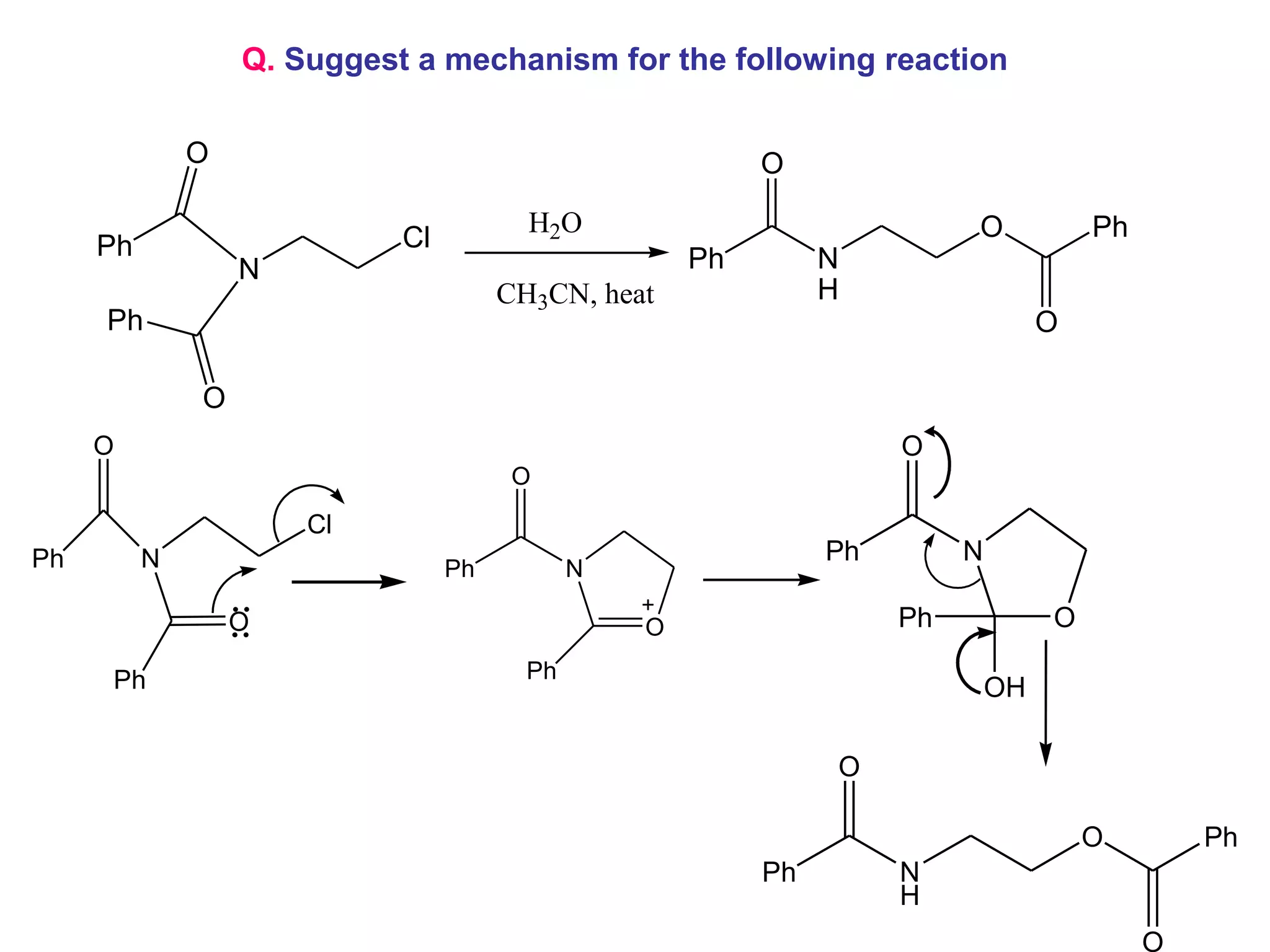
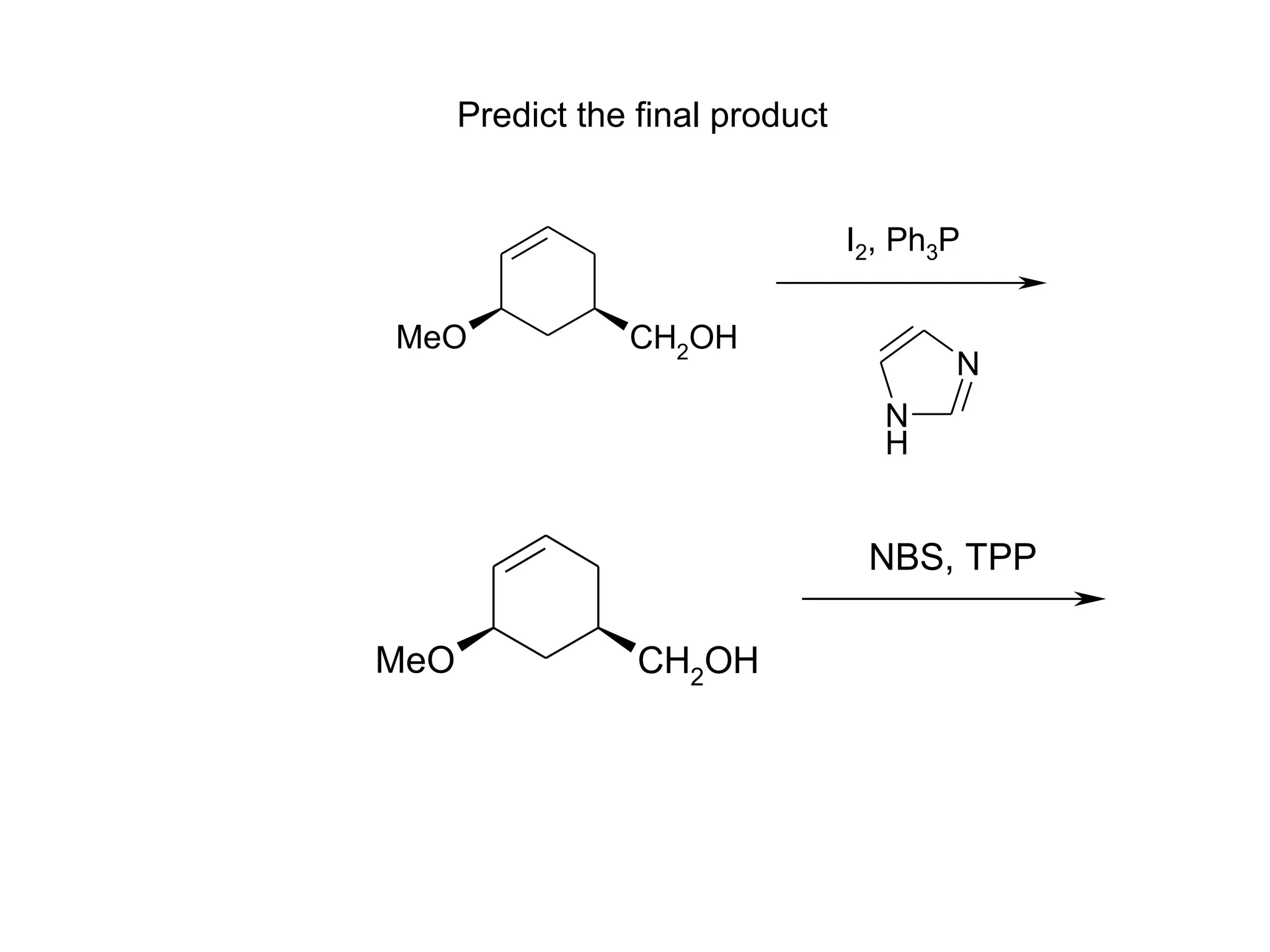
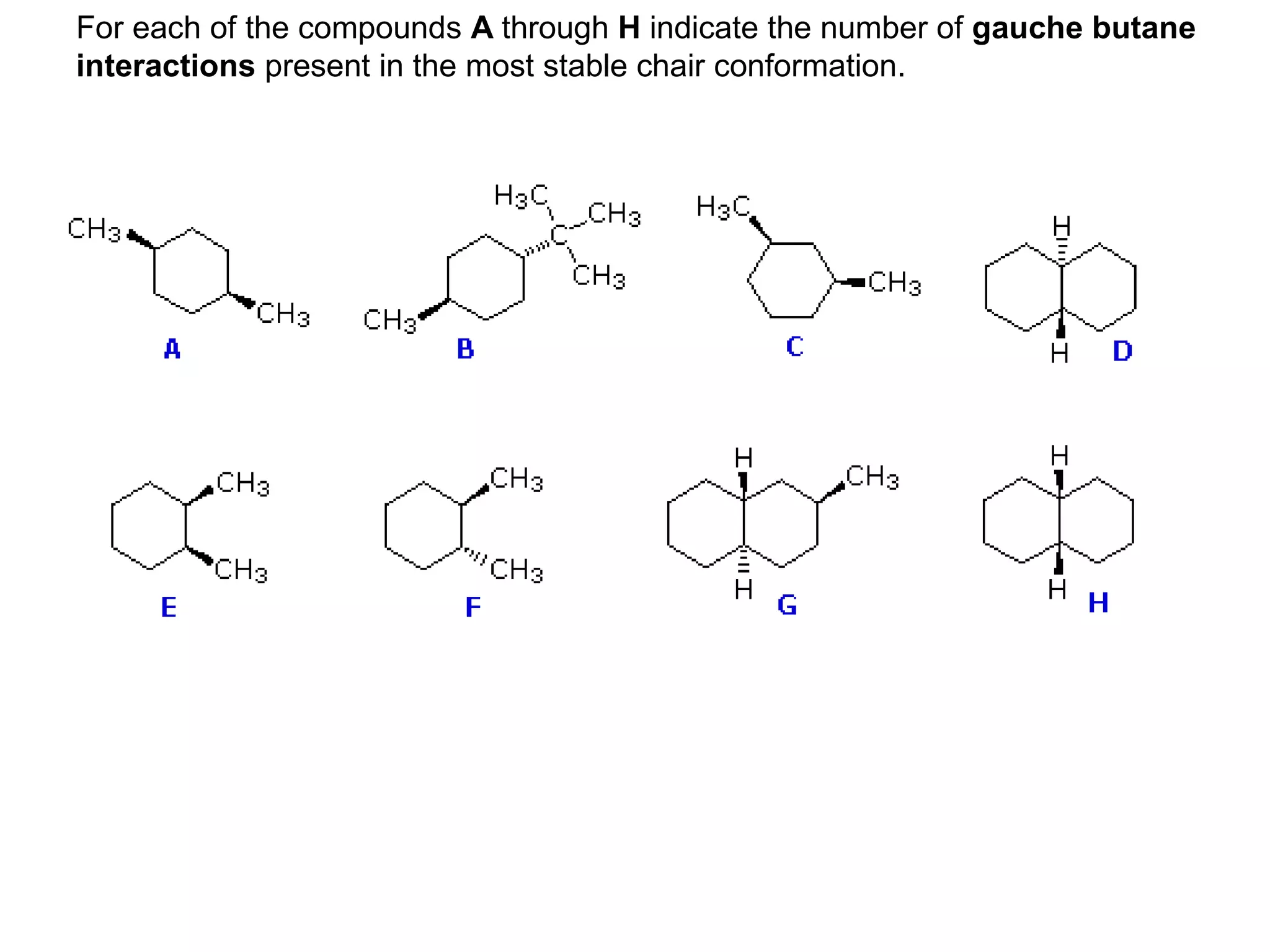
The racemic dibromide undergoes an E2 elimination with pyridine as the base to give the trans product selectively. This is because the anti-periplanar conformation required for E2 is favored for one enantiomer due to less steric interaction between the phenyl groups.
The meso dibromide cannot undergo such a stereospecific E2 reaction. Instead, it undergoes a thermally allowed homolytic cleavage of the weaker C-Br bond to eliminate Br2. This reaction does not require a particular transition state geometry.
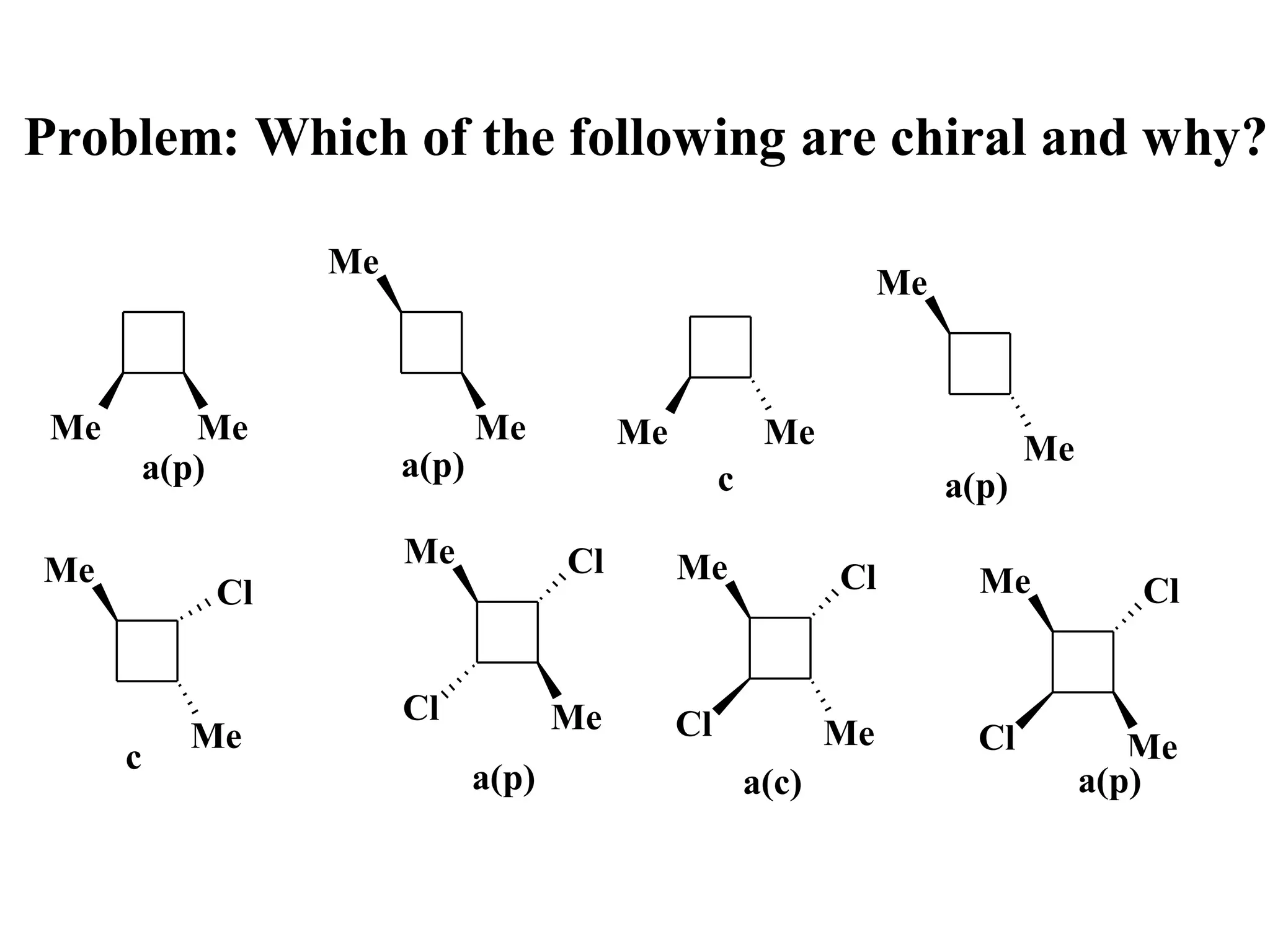
![Problem: Draw all the isomers with mol. formula C 6H-12 that
contain a cyclobutane ring, and comment on their chirality
Hint: the base structure is dimethyl cyclobutane [1,2 (cis & trans) and
1,3 (cis & trans) also 1,1]; there also exist 1ethyl cyclobutane.
Me Me Me Me
Me
Me](https://image.slidesharecdn.com/extraproblemfor1styr-130105005923-phpapp02/75/Extra-problem-for-1st-yr-7-2048.jpg)