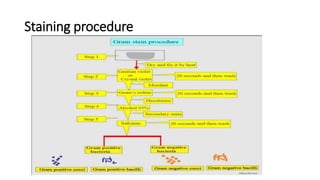
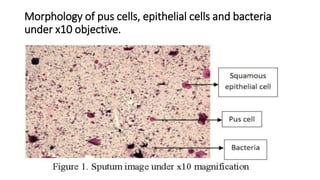
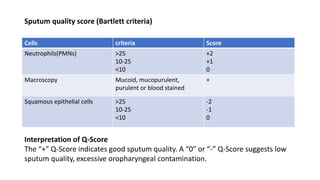
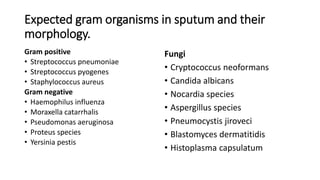
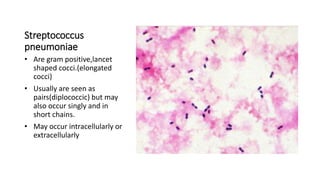
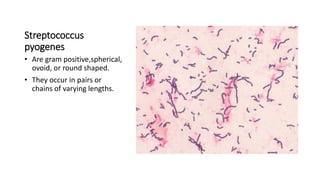
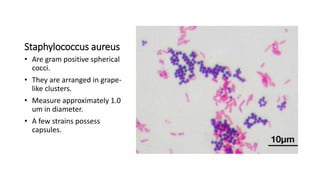
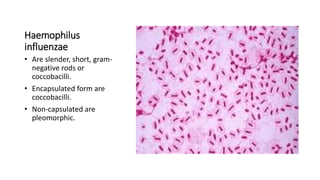
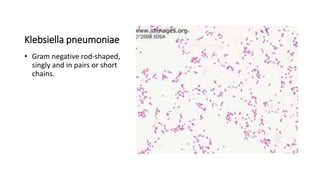
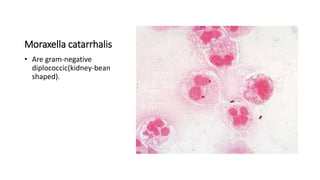
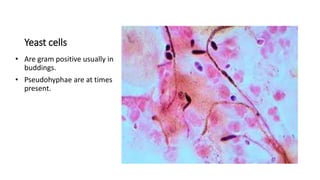
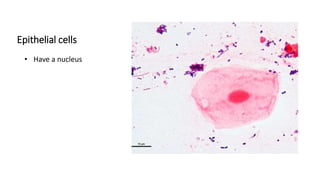
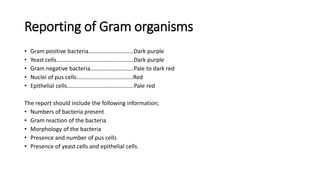
This document provides information on performing and interpreting a gram stain of sputum samples. It describes the principles behind gram staining, outlines the staining procedure and sources of errors. It also details how to evaluate sputum quality, quantify and identify bacteria, fungi and other cells seen on gram stain. Quality control measures are highlighted to help ensure accurate results.