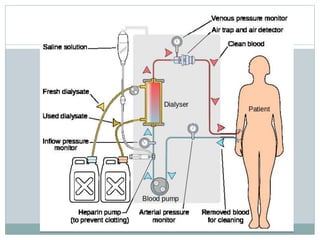
This document discusses different types of solutions and concentration expressions. It defines solution as a mixture of solute and solvent. Normal solution is defined as grams of solute dissolved in 1000 mL of solvent. Molar solution is grams of solute's molecular weight dissolved in 1000 mL. Percent solution expresses the ratio of solute to final solution weight by weight, weight by volume, or volume by volume. Osmosis and diffusion are distinguished, with osmosis being solvent movement through a semipermeable membrane towards higher solute concentration and diffusion being solute movement from higher to lower concentration. Dialysis is introduced as a process for removing waste and excess water from the blood, used as kidney replacement in failure.