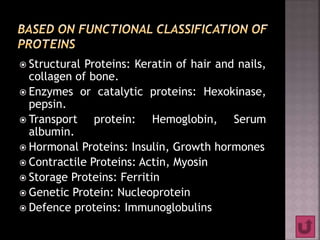
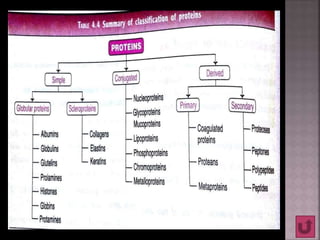
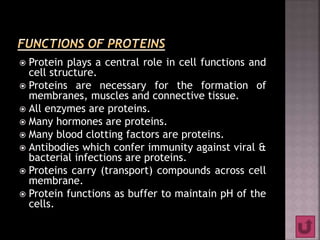
Proteins are composed of amino acids and have a complex structure ranging from primary to quaternary levels. They serve essential functions in the body such as enzyme catalysis, hormone activity, immune defenses, and structural roles. Proteins are also involved in transporting compounds and buffering pH within cells.