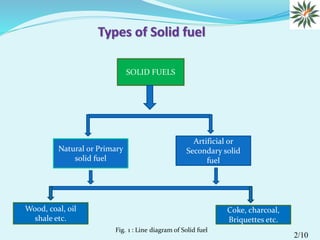
This document appears to be a seminar presentation summarizing solid fuels. It was presented by Uttam Hazarika to partially fulfill the requirements of an M.Sc. program. The presentation introduces different types of solid fuels, including primary solid fuels like wood and coal, as well as secondary solid fuels produced from coal and wood like coke, charcoal and briquettes. It discusses the advantages of solid fuels like low cost and ease of transport, as well as disadvantages like heat waste and combustion control issues. In conclusion, solid fuels have historically been important energy sources and coal remains a significant component of global fossil fuel reserves.