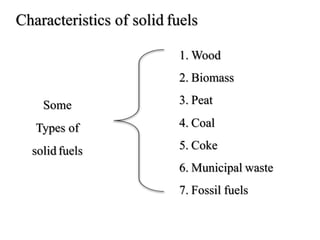
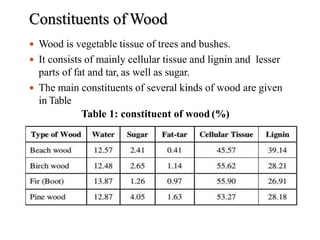
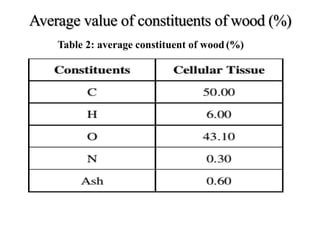
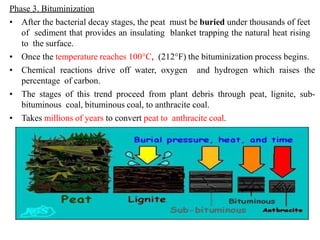
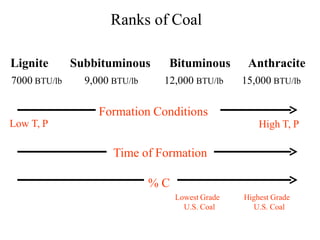
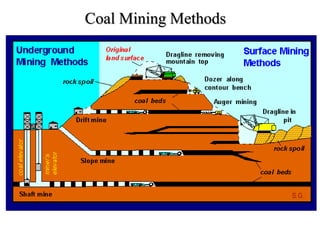
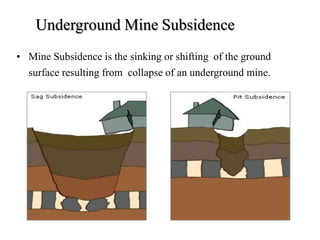
The document discusses solid fuels, defining them as materials that release energy when burned, and categorizing them into natural and manufactured types. It highlights various solid fuels such as wood, coal, and biomass, detailing their characteristics, formation processes, and classifications including lignite, sub-bituminous, bituminous, and anthracite coal. Additionally, it covers the environmental impact, mining methods, transportation logistics, and uses of solid fuels in energy production and industrial processes.