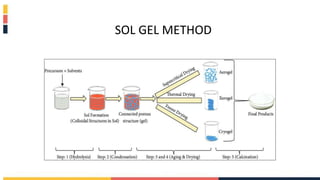
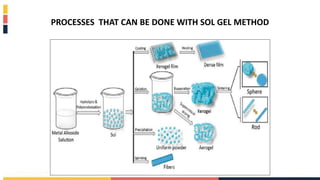
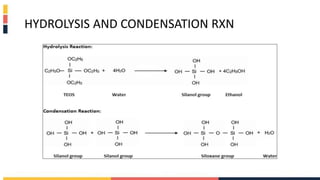
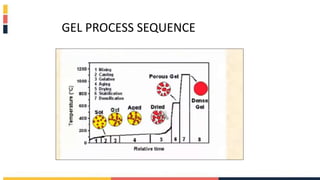
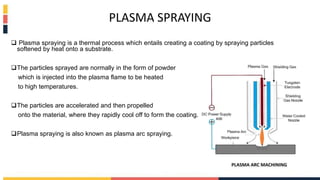
The document discusses various coating methods including sol gel, plasma spraying, and boron nitride hydrogels. The sol gel method involves converting monomers into a colloidal solution that acts as a precursor for an integrated network or gel. Plasma spraying uses high temperature plasma to heat and accelerate particles onto a substrate. Boron nitride hydrogels enhance properties like thermal conductivity and mechanical strength in hydrogels while retaining their water absorption abilities.