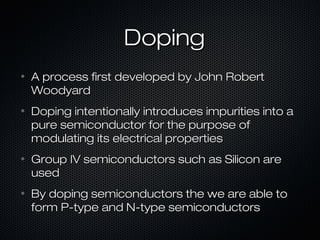
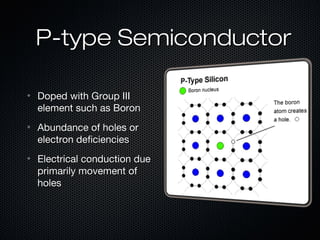
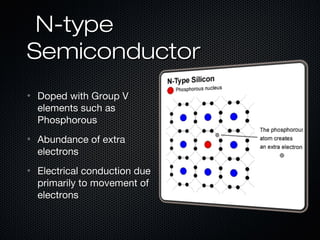
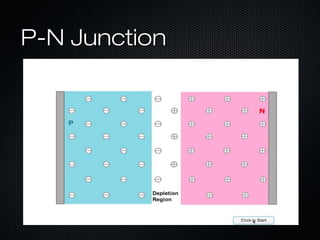
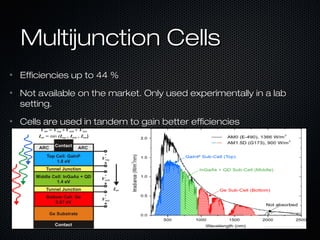
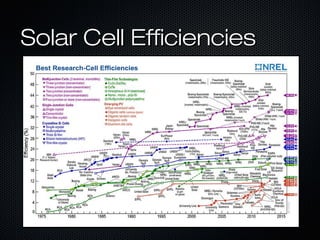
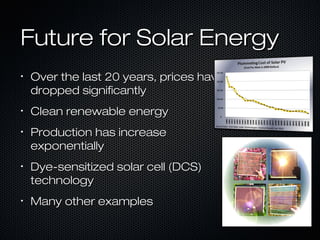
This document provides an overview of photovoltaics and solar energy. It discusses the history of photovoltaics from 1839 when the photovoltaic effect was discovered to recent developments. It describes the difference between the photovoltaic effect and photoelectric effect. It also explains how semiconductors are produced through doping to create P-N junctions and the different types of solar cells including thin film, polycrystalline and monocrystalline cells. The document concludes with a look at future developments in solar energy technology.