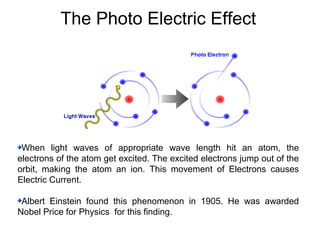
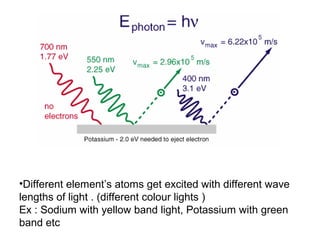
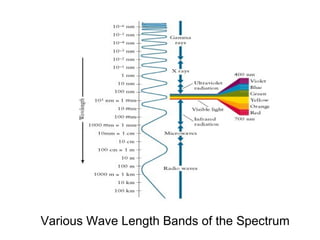
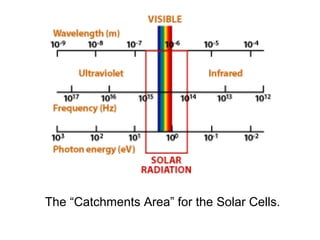
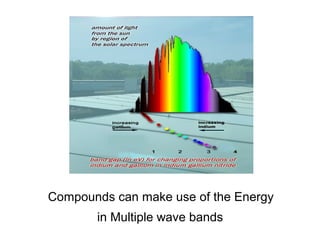
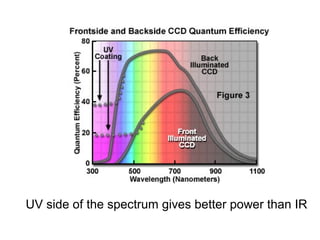
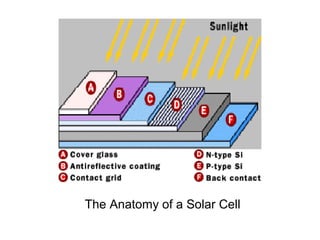
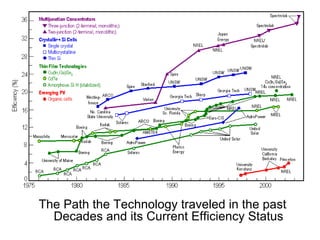
Solar cells convert sunlight into electricity by using the photoelectric effect where electrons are excited by photons and produce an electric current. Albert Einstein discovered this phenomenon in 1905 and won the Nobel Prize for it. Solar cells use materials that absorb different wavelengths of light to generate electricity, with compounds able to use multiple bands for greater efficiency. While efficiency has improved over decades of research, more advancement is still needed to perfect solar cell technology.