Embed presentation
Download to read offline


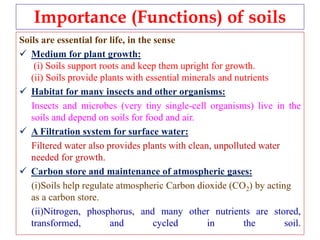
![pH of soil
pH is a measure of hydrogen ion concentration, a measure
of the acidity or alkalinity of a solution.
pH = -log [H+]
The pH scale ranges from 0 to 14, with pH 7.0 being
neutral. pH less than 7 are acidic, while those with a pH
greater than 7 are basic or alkaline.
The optimal pH range for most plants is between 5.5
and 7.0; however, many plants have adapted to thrive
at pH values outside this range.](https://image.slidesharecdn.com/soilpollution-200426071940/85/Soil-Pollution-4-320.jpg)
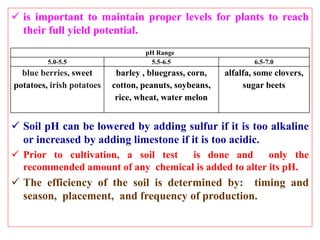

Soil pollution occurs when persistent toxic substances accumulate in soil, harming plant growth and animal health. Soils are essential as they support plant roots and provide nutrients, serve as a habitat for insects and microbes, filter surface water, store carbon to regulate climate, and cycle nutrients. The pH or acidity level of soil must be maintained within a suitable range, typically between 5.5-7, for most plants to thrive, through adding limestone to raise pH or sulfur to lower it. A soil test determines amendments needed to optimize soil conditions for crop yields.



![pH of soil
pH is a measure of hydrogen ion concentration, a measure
of the acidity or alkalinity of a solution.
pH = -log [H+]
The pH scale ranges from 0 to 14, with pH 7.0 being
neutral. pH less than 7 are acidic, while those with a pH
greater than 7 are basic or alkaline.
The optimal pH range for most plants is between 5.5
and 7.0; however, many plants have adapted to thrive
at pH values outside this range.](https://image.slidesharecdn.com/soilpollution-200426071940/85/Soil-Pollution-4-320.jpg)

