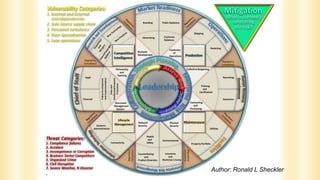
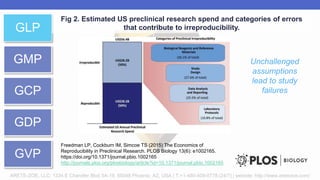
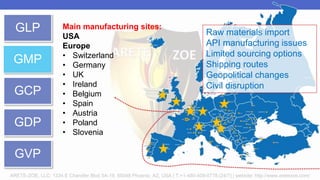
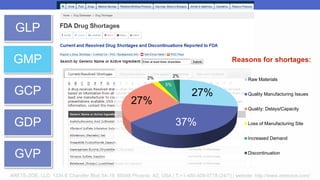
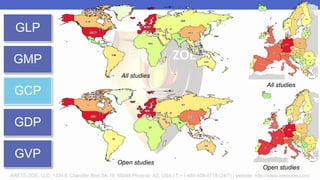
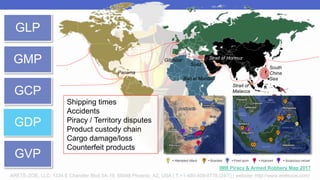
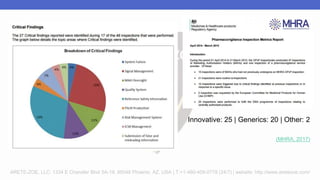
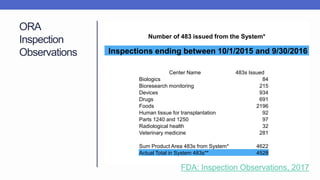
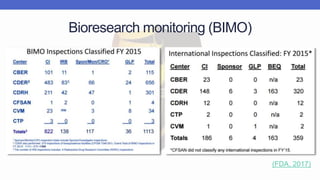
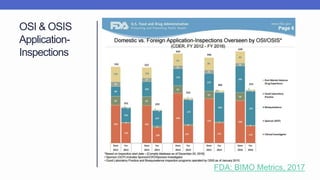
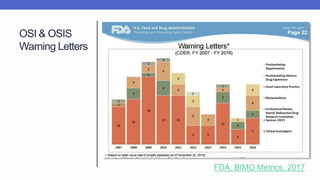
Arete-Zoe is a consulting firm that provides services related to risk management in the pharmaceutical industry. They help clients address issues in clinical research, healthcare systems, and public health. Arete-Zoe analyzes vulnerabilities and provides recommendations to improve organizational effectiveness, business resiliency, and safety. Their services include assessing risks from manufacturing and supply chain issues, inspections, clinical trials, and other factors that can undermine the pharmaceutical industry.