Systems engineering is an interdisciplinary approach that focuses on defining customer needs, documenting requirements, and enabling the realization of successful systems. It considers both business and technical needs across the entire life cycle from concept to disposal. Requirements management is the foundation of systems engineering. Organizations can improve processes and reduce risks through structured approaches like the Systems Engineering V-Model and maturity models like CMMI that provide standard processes and best practices. Verification and validation are used to ensure a system meets its requirements through methods like testing, analysis and demonstration.


.[1]
What is Systems Engineering?
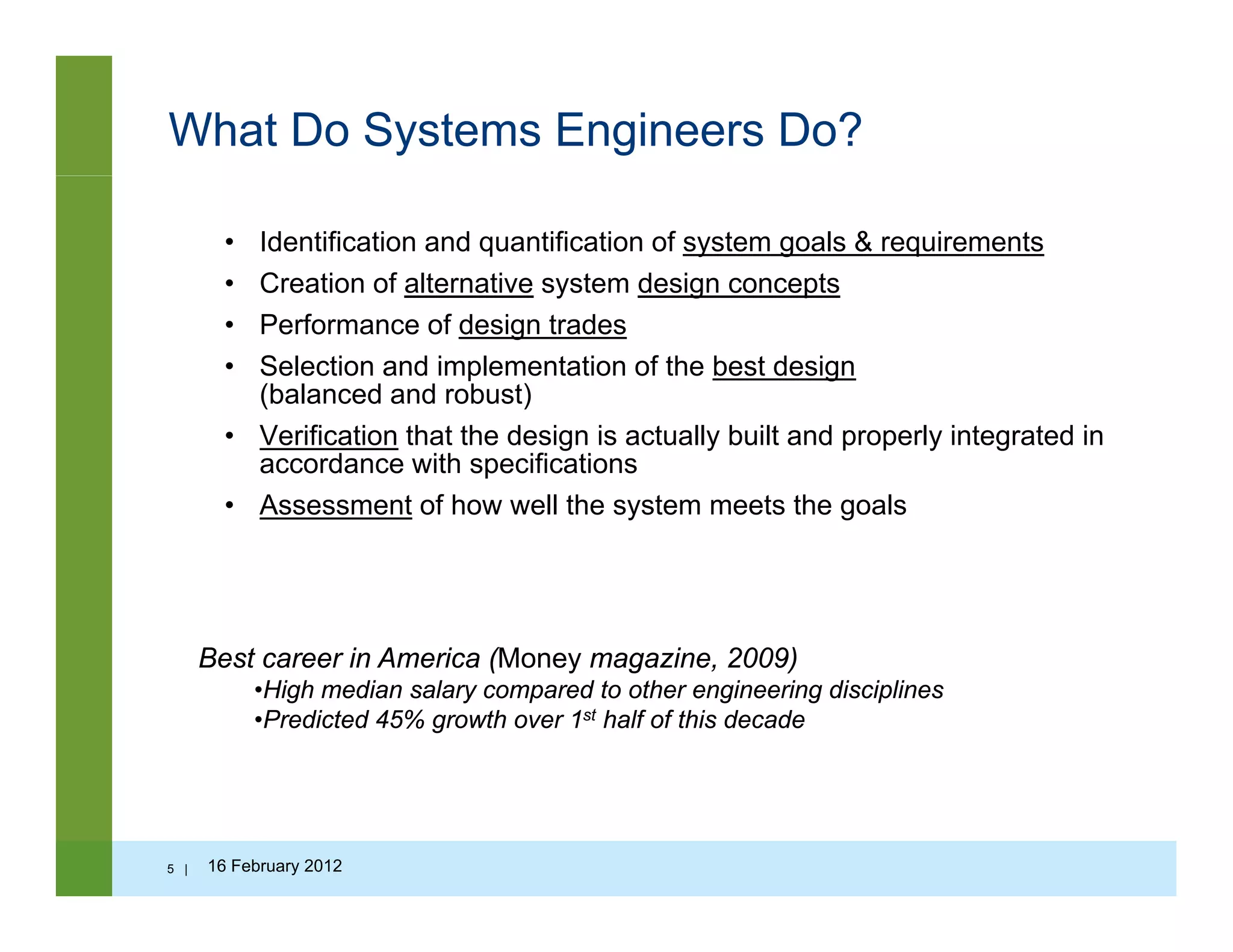
Systems engineering is an iterative process of top-down synthesis, development, and
operation of a real-world system that satisfies in a near optimal manner the full range ofoperation of a real world system that satisfies, in a near optimal manner, the full range of
requirements for the system. (Eisner)
Systems Engineering is an interdisciplinary approach and means to enable the realization
of successful systems. It focuses on defining customer needs and required functionalityy g q y
early in the development cycle, documenting requirements, and then proceeding with
design synthesis and system validation while considering the complete problem:
operations, cost and schedule performance, training and support, test, manufacturing, and
disposal. SE considers both the business and technical needs of all customers with the
l f idi li d h h d (INCOSE)goal of providing a quality product that meets the user needs.(INCOSE)
Differs from other specialist disciplines of engineering, focus on technical coordinationDiffers from other specialist disciplines of engineering, focus on technical coordination
4 | 16 February 2012](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-4-2048.jpg)
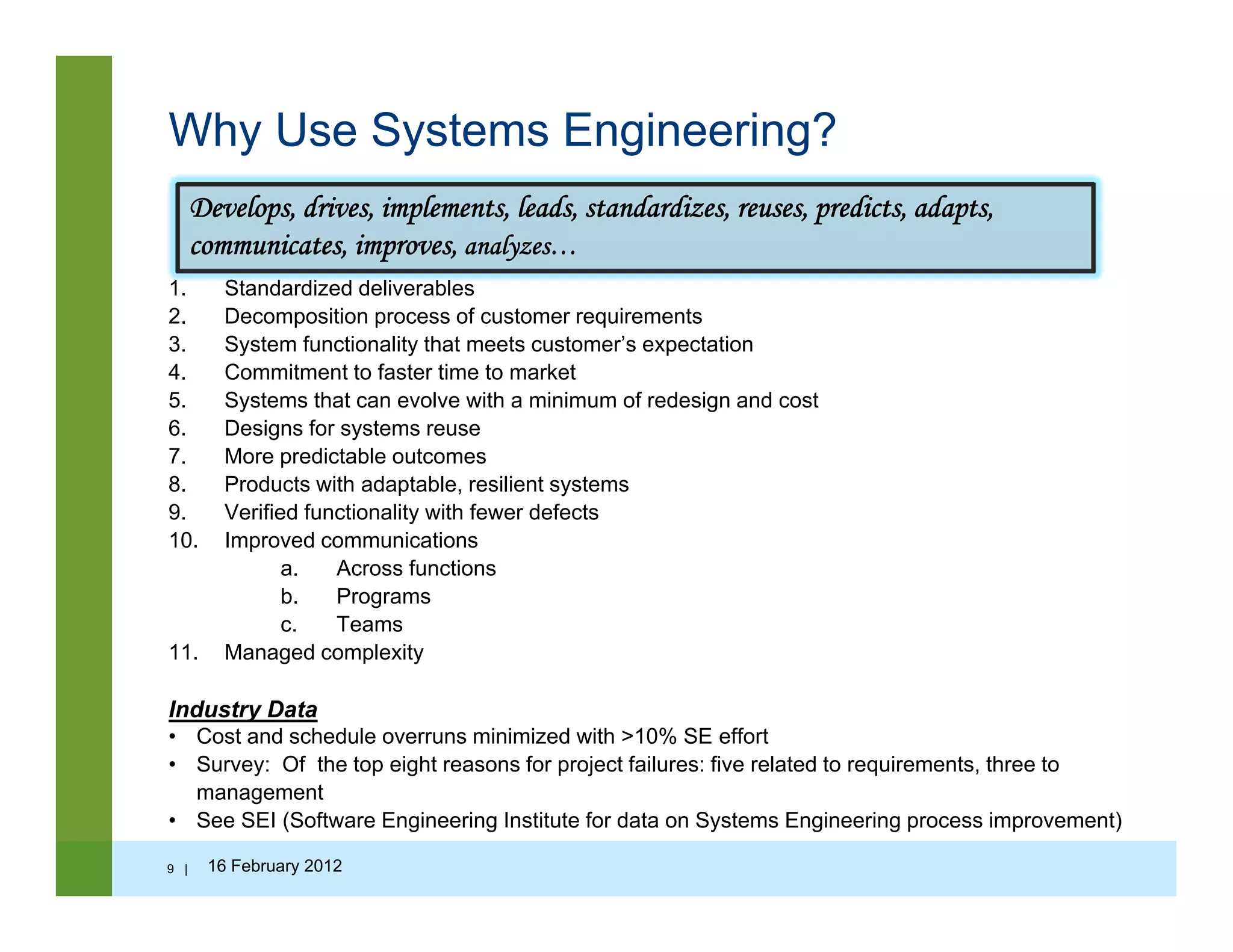
![Why Do We Need Systems Engineering?
Competitive pressures from the rapid advancement of integrated technologies
• Increased product complexity
• Reduction of product development cycle time
I d f t d l t i t• Increased safety and regulatory requirements
• Globalization of the marketplace and workforce
• Software as a dominant force of change in new products
• Worldwide deployment of new technology on ever-shorter time scales
S t t t d f i ti t i t ll t l t• Systems constructed from pre-existing components or intellectual property
• Re-use of components, information, and knowledge across projects
• Transition from paper-based control to electronically managed information
• The rise of intellectual capital as the primary asset of many modern organizations
6 | 16 February 2012
INCOSE Handbook, [2]](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-6-2048.jpg)

![Systems Thinking
Definition:
The process of understanding how things influence one another
within a wholewithin a whole
Foundation in the field of system dynamics by Jay Forester in 1956 at MIT
• Applying engineering principles to social systems
• Study interactions vs decomposition and constituent analysis
Basic Tenets
• Interdependence of objects and their attributes - independent elements can never constitute a system
• Holism - emergent properties not possible to detect by analysis should be possible to define by a holistic
approach
G f• Goal seeking - systemic interaction must result in some goal or final state
• Inputs and Outputs - in a closed system inputs are determined once and constant; in an open system
additional inputs are admitted from the environment
• Transformation of inputs into outputs - this is the process by which the goals are obtained
• Entropy - the amount of disorder or randomness present in any system• Entropy - the amount of disorder or randomness present in any system
• Regulation - a method of feedback is necessary for the system to operate predictably
• Hierarchy - complex wholes are made up of smaller subsystems
• Differentiation - specialized units perform specialized functions
• Equifinality - alternative ways of attaining the same objectives (convergence)q y y g j ( g )
• Multifinality - attaining alternative objectives from the same inputs (divergence)
8 | 16 February 2012
Weinberg, [4]](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-8-2048.jpg)

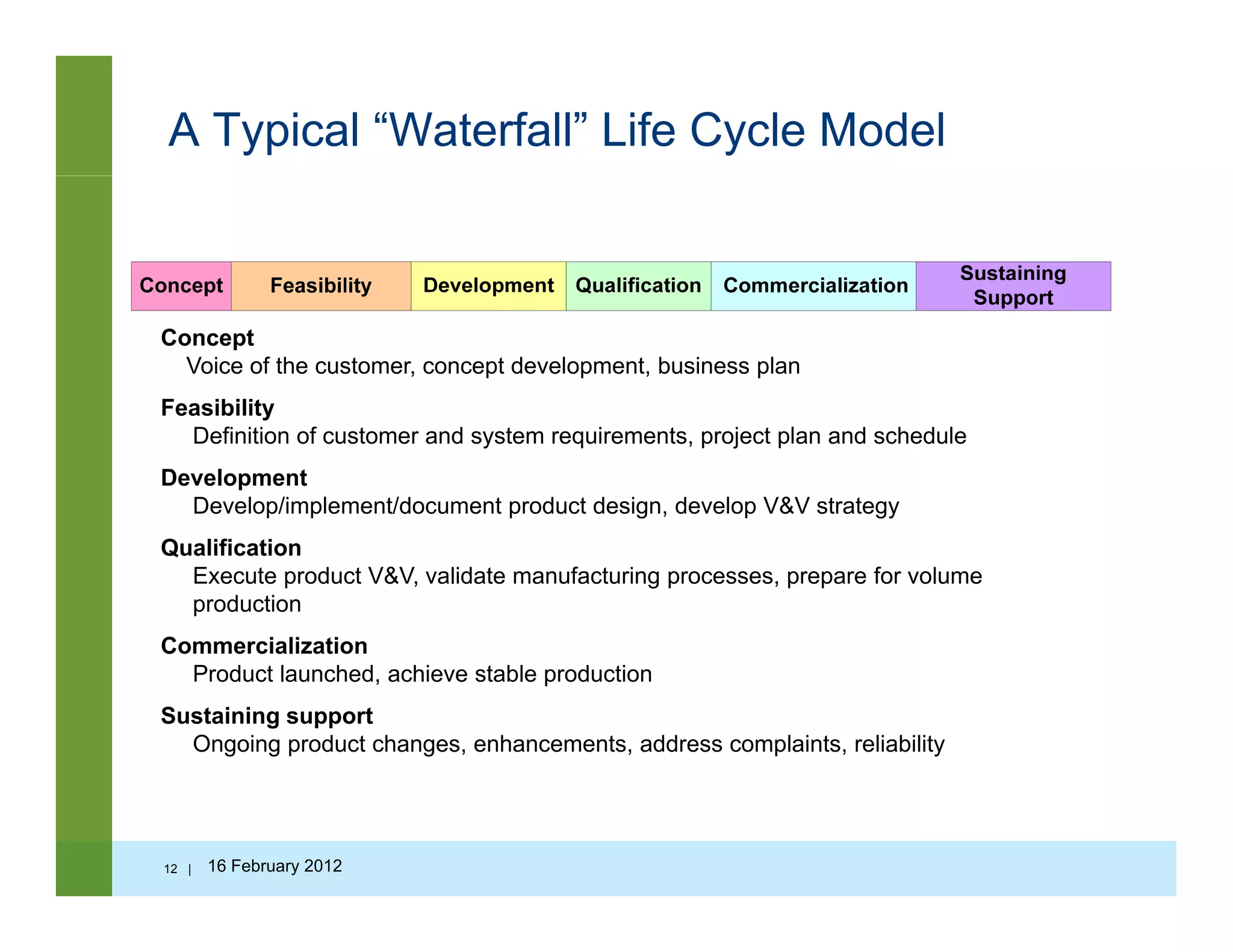
![The Systems Engineering V Model
User
requirements
Validation
Tests
Validation
System
Requirements
System Tests
Verification
Requirements
IntegrationArchitectural
VerificationDefining the
d t
Integrating
& verifying
what has
been b ilt
g
TestsDesign
product been built
Abstract,
early, formative,
creative, conducive
Systems Engineering
Component Engineering
Component
Development
Component
Tests
to change
Expensive, realistic,
late, difficult to change
Component Engineering
13 | 16 February 2012
Stevens, [6]](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-13-2048.jpg)
![ISO /IEC 15288: 2008 Systems and Software
E i i S t Lif C l PEngineering – Systems Life Cycle Processes
14 | 16 February 2012
INCOSE Handbook, [2]](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-14-2048.jpg)


![CIMM – The Missing Levels (Humor)
0 : Negligent0 : Negligent
The organization pays lip service, often with excessive fanfare, to implementing software engineering
processes, but lacks the will to carry through the necessary effort. Whereas CMM level 1 assumes
eventual success in producing software, CIMM level 0 organizations generally fail to produce any
product, or do so by abandoning regular procedures in favor of crash programs.product, or do so by abandoning regular procedures in favor of crash programs.
-1 : Obstructive
Processes, however inappropriate and ineffective, are implemented with rigor and tend to obstruct work.
Adherence to process is the measure of success in a Level -1 organization. Any actual creation of viable
product is incidental. The quality of any product is not assessed, presumably on the assumption that if
the proper process was followed, high quality is guaranteed.
Paradoxically, Level -1 organizations believe fervently in following defined procedures, but lacking the
will to measure the effectiveness of the procedures they rarely succeed at their basic task of creating
software.
2 C t t-2 : Contemptuous
While processes exist, they are routinely ignored by engineering staff and those charged with overseeing
the processes are regarded with hostility. Measurements are fudged to make the organization look good.
This is not a good environment to work in or be associated with.
-3 : Undermining-3 : Undermining
Not content with faking their own performance, undermining organizations routinely work to downplay
and sabotage the efforts of rival organizations, especially those successfully implementing processes
common to CMM level 2 and higher. This is worst where company policy causes departments to
compete for scarce resources, which are allocated to the loudest advocates.p ,
Schorsch, [7]
20 | 16 February 2012](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-20-2048.jpg)



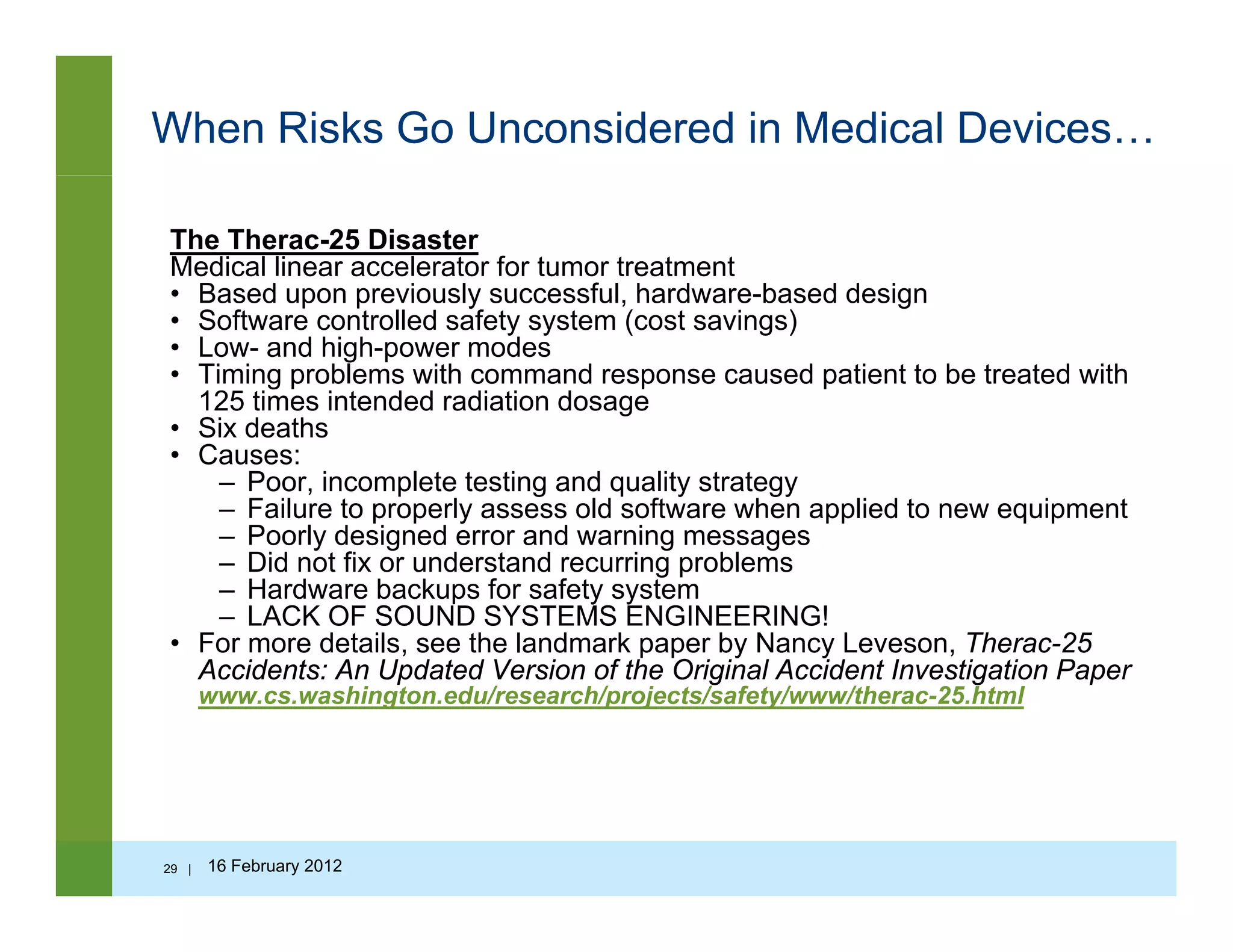



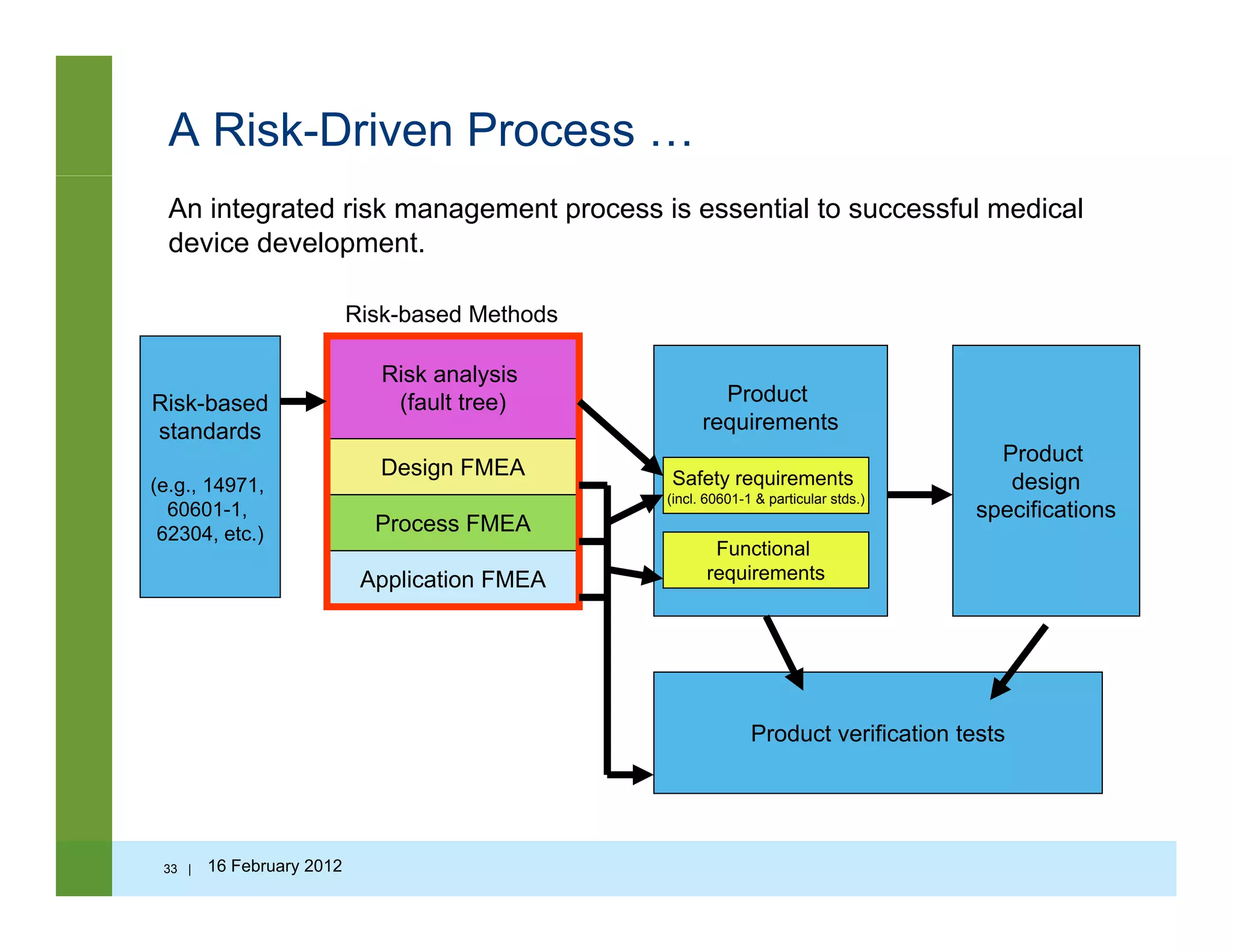


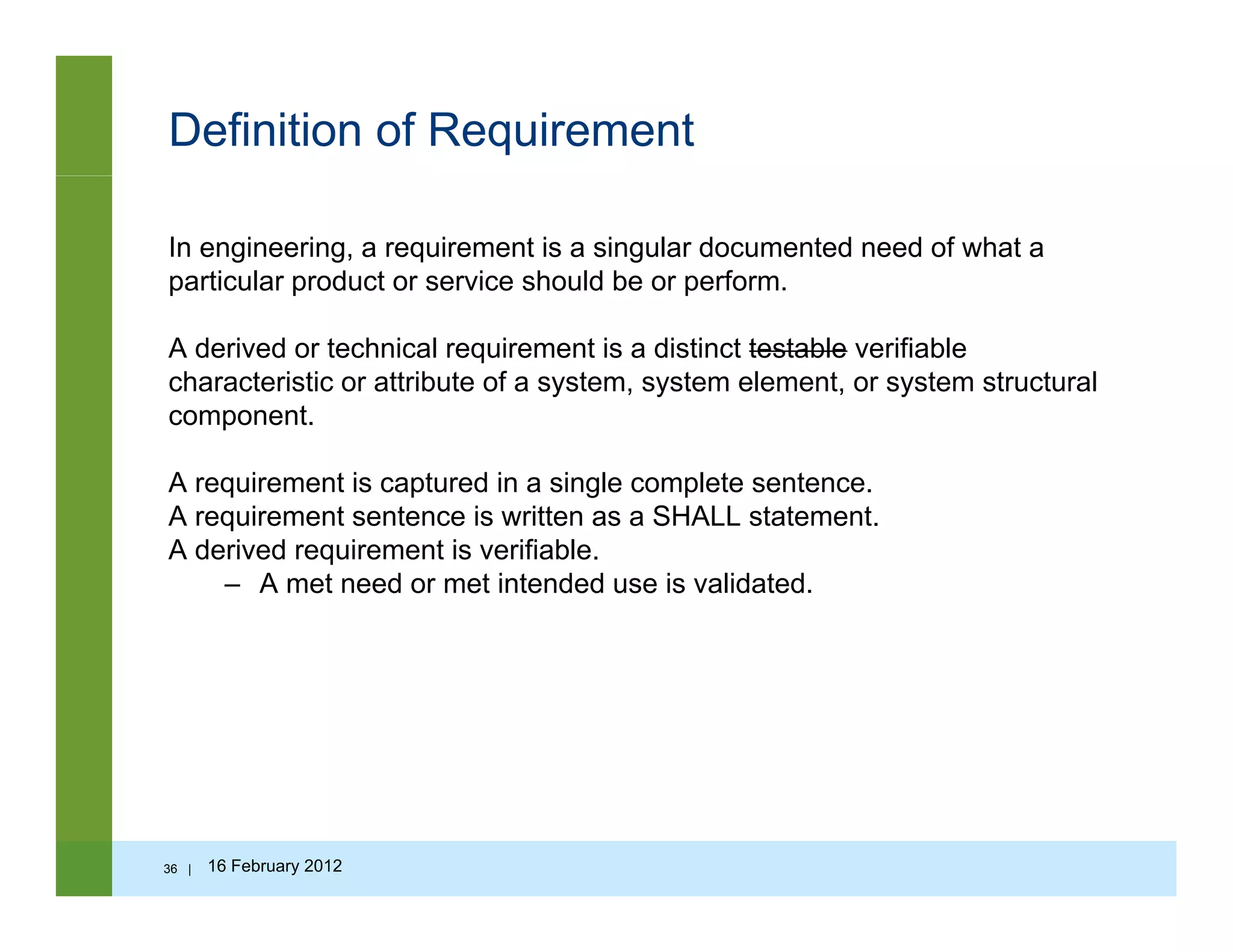


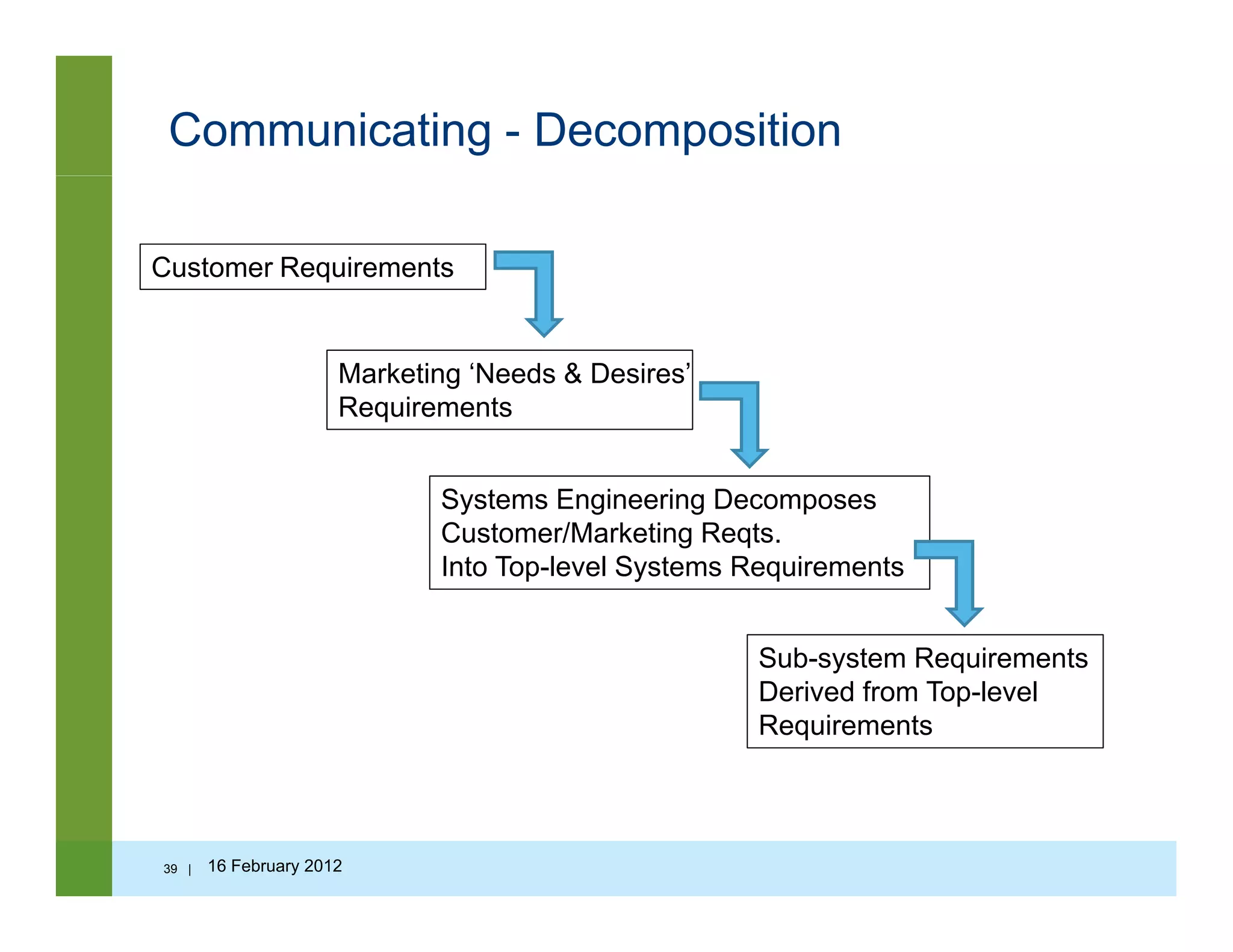




![Questions to ask…
• Is the requirement correct?
• Is the requirement verifiable?• Is the requirement verifiable?
• Is the requirement clear?
• Is the requirement consistent?
• Is the requirement feasible?
47 | 16 February 2012
Telelogic DOORS, [8]](https://image.slidesharecdn.com/systemsengineeringandrequirementsmanagementinmedicaldeviceproductdevelopment-130711145840-phpapp01/75/Systems-Engineering-and-Requirements-Management-in-Medical-Device-Product-Development-47-2048.jpg)