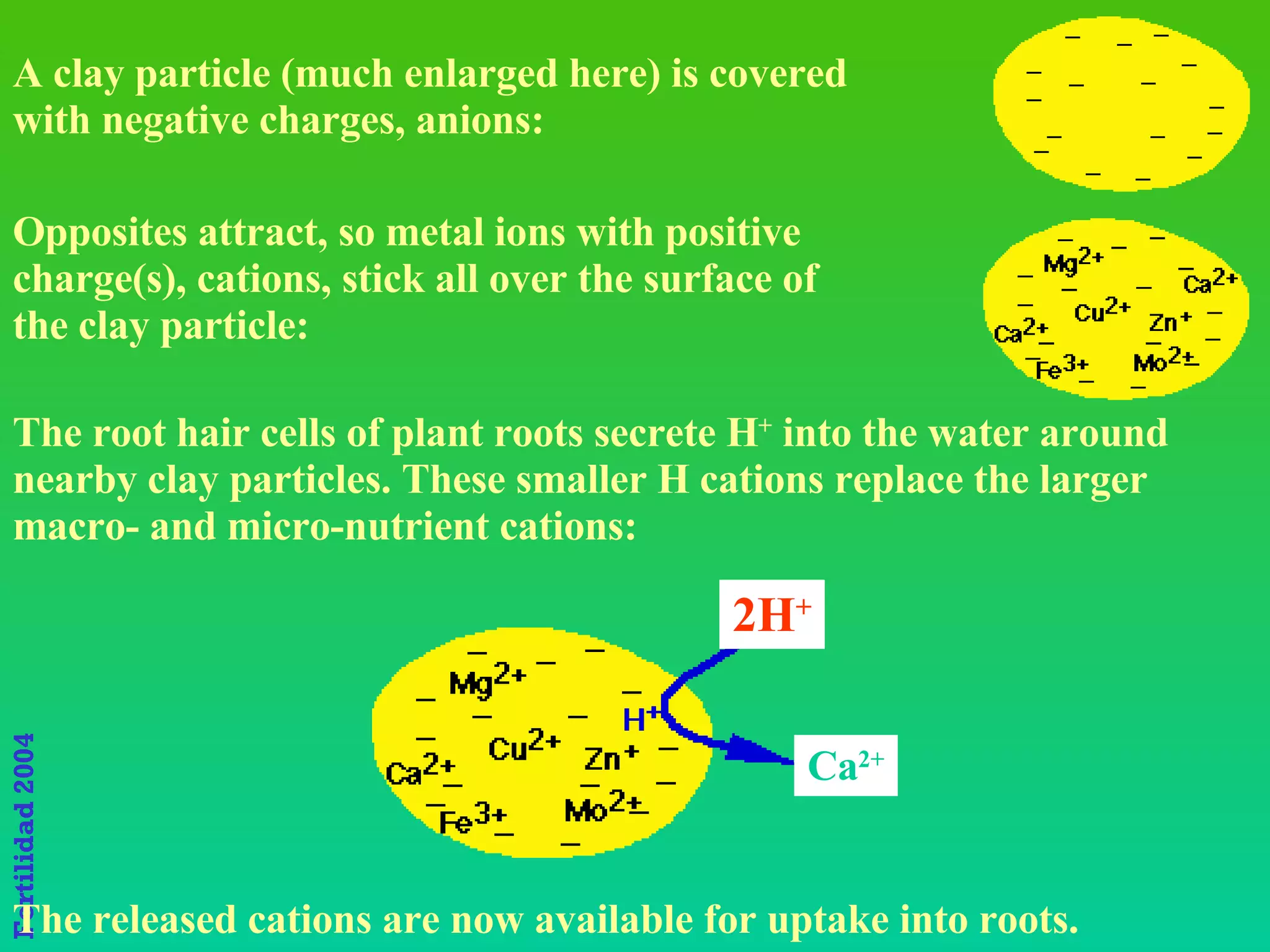
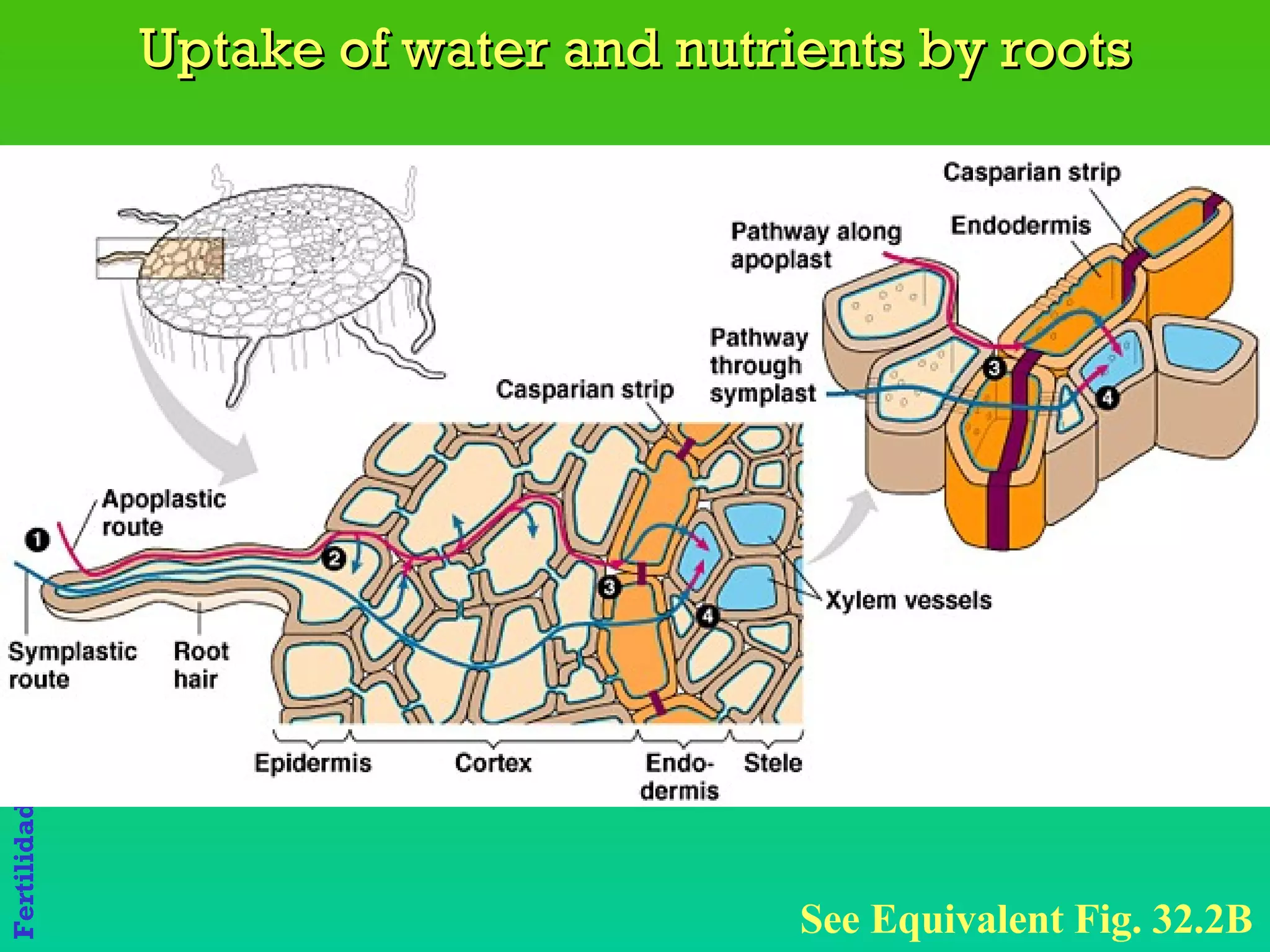
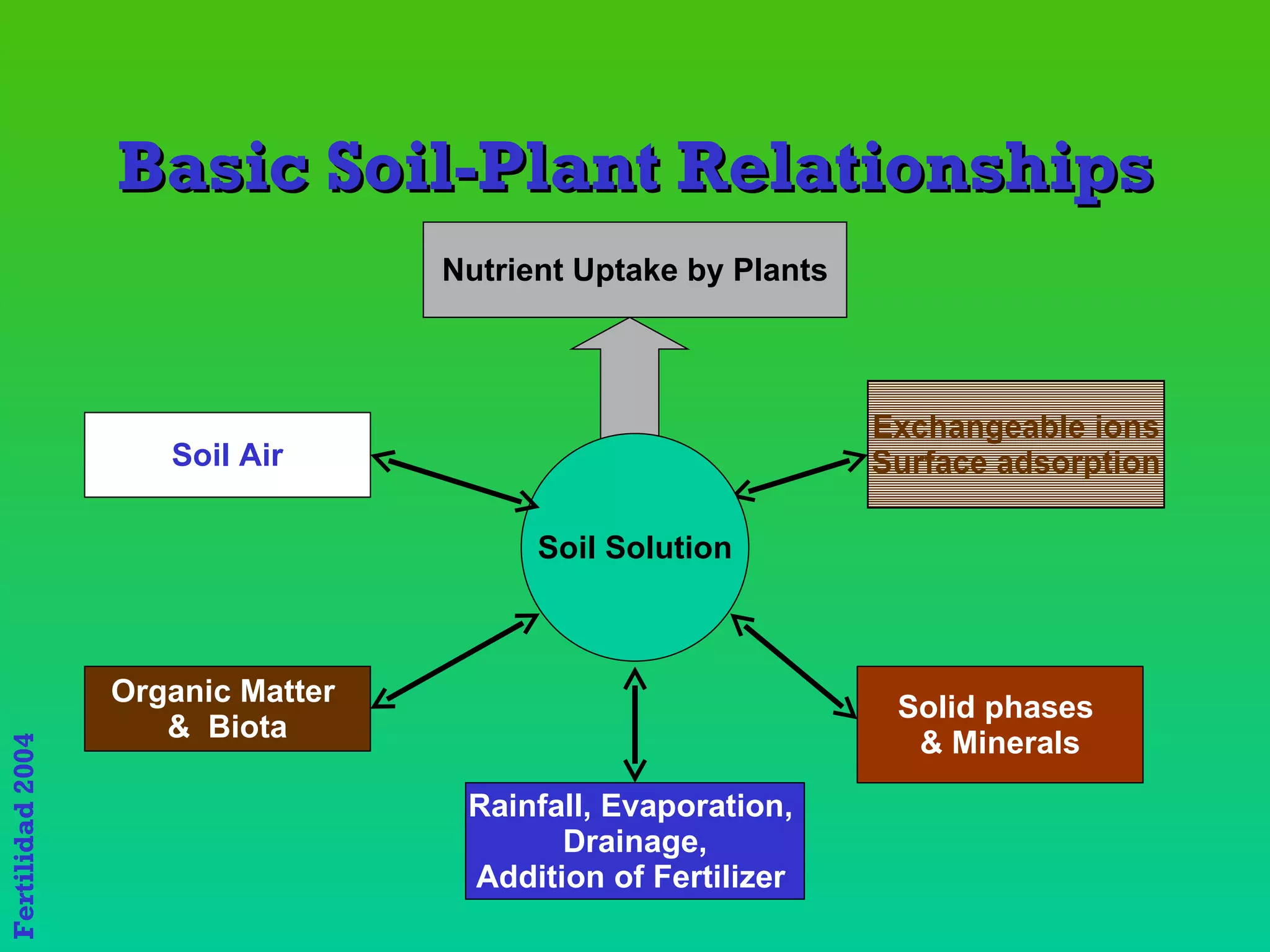
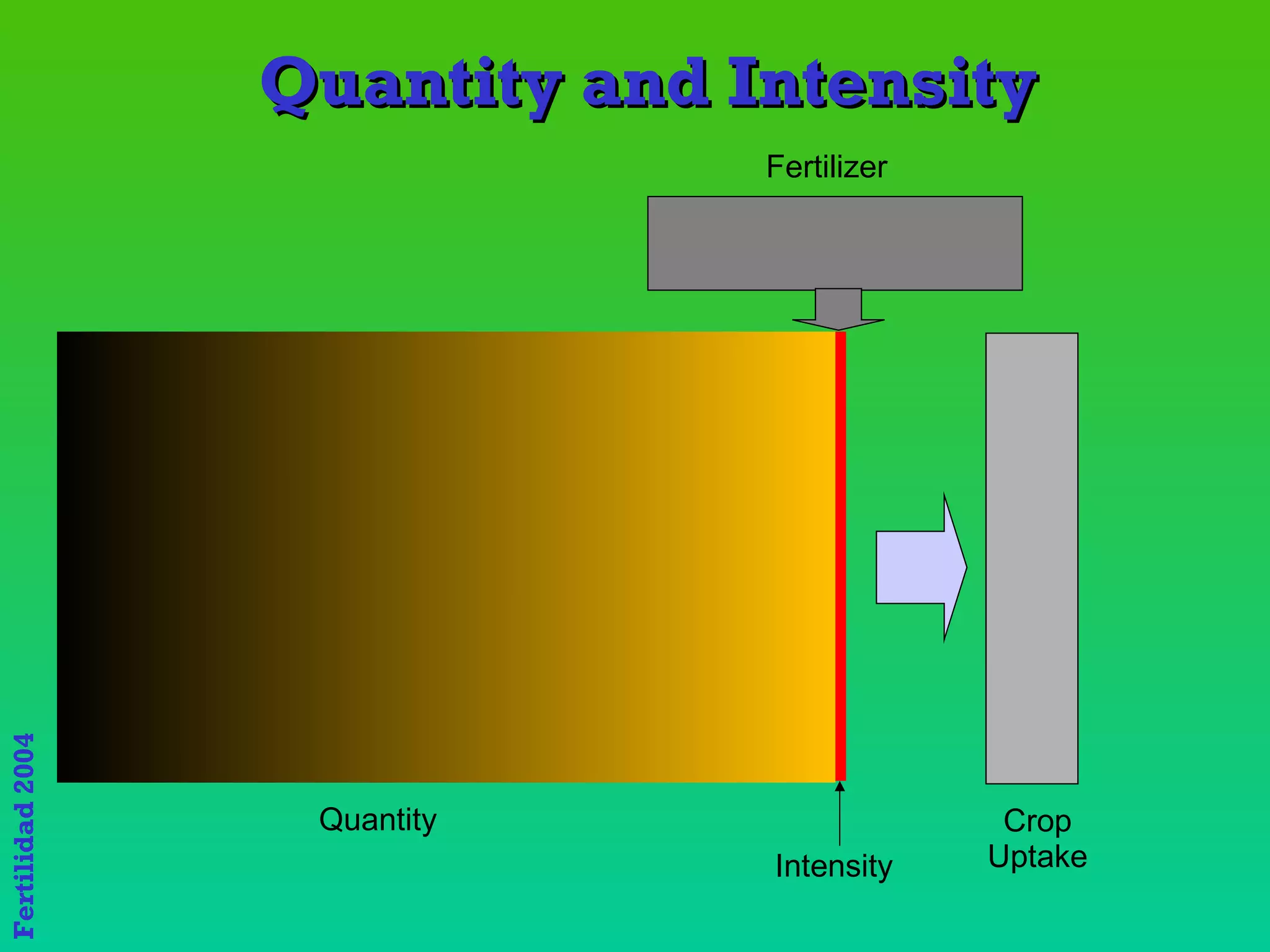
The document discusses the close link between humans and soil. It notes that the name "Adam" comes from the Hebrew word "Adamiss" meaning earth or soil, and his life and livelihood derive from the soil. It also notes that Eve's name, "Hava", literally means life. Together Adam and Eve signify "Soil and Life," highlighting the inextricable relationship between humans and the land.
















![Mineral soils The concentration of dissociated water in freshly-distilled water is 10 -7 M. This is used to describe acidity-alkalinity, originally called the pouvoir Hydrogéne, which we know now as pH. ACIDEZ DEL SUELO determinará CÓMO estarán disponibles Nutrientes disponibles a través del AGUA DEL SUELO Small quantities of water molecules dissociate : pH = - log [H + ] = - log [10 -7 M] = 7 for fresh distilled water Small values for acid , e.g., the water in Sphagnum bogs can be ~3 Large values for alkaline , e.g., soils on limestone ~8 Suelos Minerales H 2 O OH - + H +](https://image.slidesharecdn.com/pfertilidad-clase-1-2008-1219975469599806-9/75/P-Fertilidad-Clase-1-2008-18-2048.jpg)