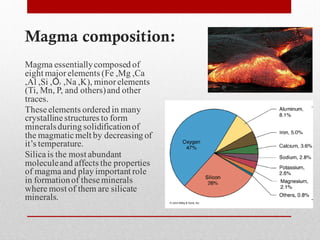
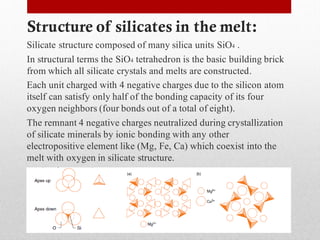
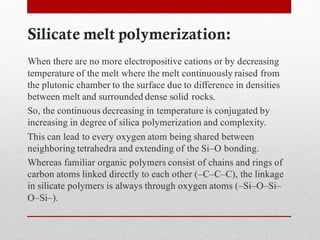
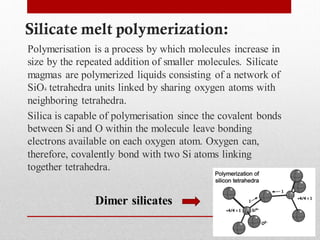
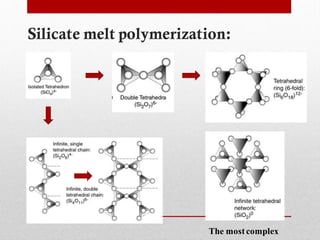
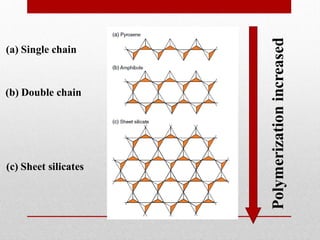
1) Silicate polymerization occurs as magma cools and silica content increases, linking silica tetrahedra units through shared oxygen atoms.
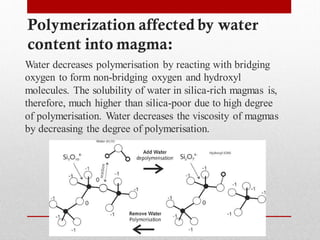
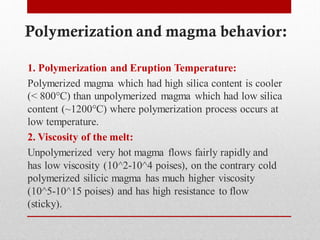
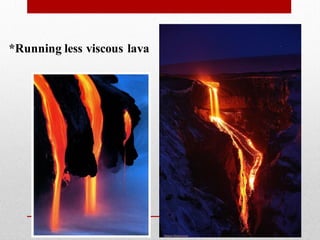
2) More polymerized magma has higher viscosity and lower temperature than unpolymerized magma. It also contains more volatiles.
3) The degree of polymerization affects physical properties of magma like viscosity and eruption behavior, with highly polymerized magma producing explosive eruptions due to high volatile content.





![For further reading:
This reference is recommended:
Chemical Fundamentalsof Geology and
EnvironmentalGeoscienceby [Robin
Gill , 2015]3rd edition.
Reference downloadinglink:
ha9ll3si6http://www.mediafire.com/download/
/Chemical+Fundamentals+of+Geology+62chp2
BRobin5and+Environmental+Geoscience++%
-Geo+Pedia+Geo28D+%5%2015C+2+Gill+%
.pdf29Library%](https://image.slidesharecdn.com/silica-polymerization-in-igneous-processes-160212002243/85/Silica-polymerization-in-igneous-processes-16-320.jpg)
