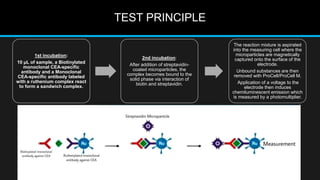
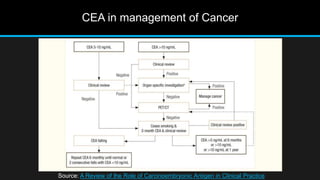
The document discusses carcinoembryonic antigen (CEA), an oncofetal antigen utilized as a tumor marker for monitoring cancer treatment and recurrence. It outlines its biological characteristics, diagnostic methods, and the implications of elevated CEA levels in both malignant and benign conditions. Recommendations for CEA testing procedures and the significance of CEA levels in various patient demographics are also highlighted.