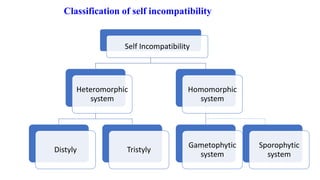
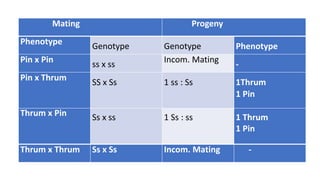
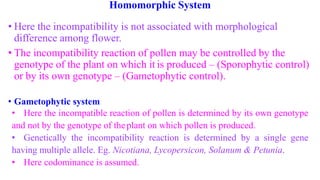
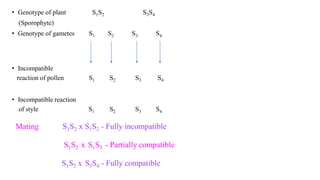
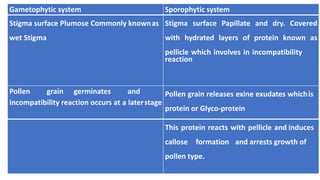
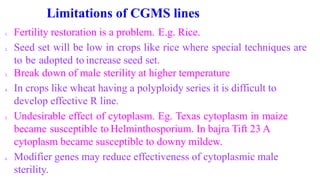
The document discusses self-incompatibility and male sterility in plants and their use in crop improvement. It describes different types of self-incompatibility systems including heteromorphic, homomorphic gametophytic and sporophytic systems. It also discusses the mechanisms of self-incompatibility at the pollen-stigma, pollen tube-style and pollen tube-ovule levels. Furthermore, it outlines different types of male sterility including cytoplasmic, genetic, and transgenic systems and their uses in hybrid seed production. Maintaining male sterile lines and developing effective fertility restoration systems are also challenges.