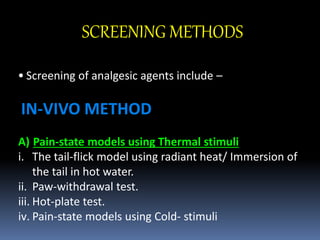
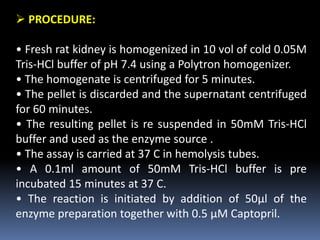
This document provides an overview of screening methods for analgesic drugs. It discusses various in vivo and in vitro methods used to screen analgesics, including pain-state models using thermal, mechanical, electrical, and chemical stimuli in animals. Specific in vivo models described are the tail-flick test, hot-plate test, acetic acid-induced writhing test, and various electrical and chemical stimulation tests. In vitro methods discussed include bioassays using isolated tissues to study nociceptin receptors, radioligand binding assays like 3H-naloxone binding to study opioid agonists/antagonists, and inhibition of enkephalinase as a screening method.